|
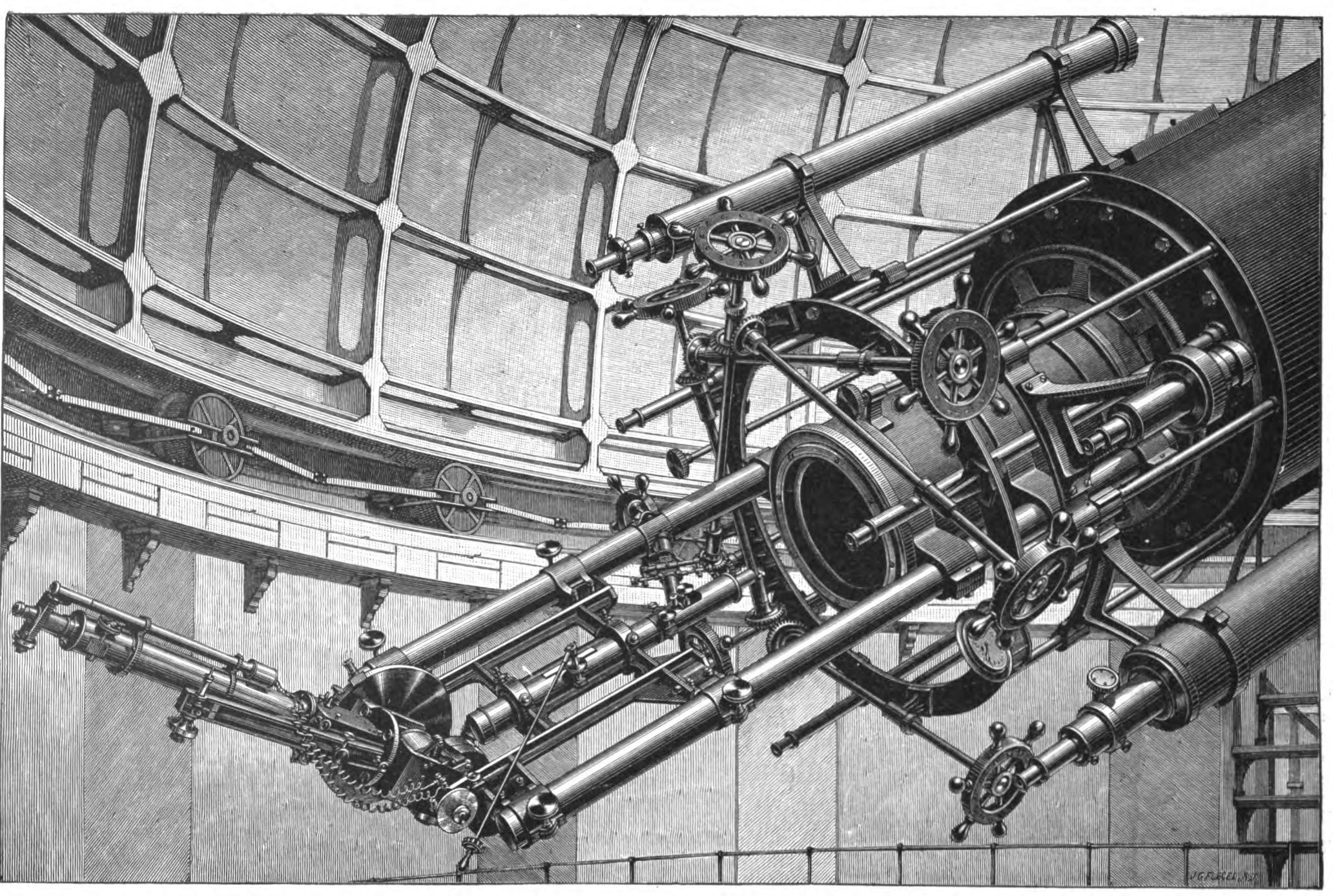
Spectrometer
A spectrometer () is a scientific instrument used to separate and measure Spectrum, spectral components of a physical phenomenon. Spectrometer is a broad term often used to describe instruments that measure a continuous variable of a phenomenon where the spectral components are somehow mixed. In visible light a spectrometer can separate white light and measure individual narrow bands of color, called a spectrum. A mass spectrometer measures the spectrum of the masses of the atoms or molecules present in a gas. The first spectrometers were used to split light into an array of separate colors. Spectrometers were History_of_spectroscopy, developed in early studies of physics, astronomy, and chemistry. The capability of spectroscopy to determine Analytical_chemistry#Spectroscopy, chemical composition drove its advancement and continues to be one of its primary uses. Spectrometers are used in Astronomical spectroscopy, astronomy to analyze the chemical composition of Astronomical_spe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Spectrometer
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures. A mass spectrum is a type of plot of the ion signal as a function of the mass-to-charge ratio. These spectra are used to determine the elemental or isotopic signature of a sample, the masses of particles and of molecules, and to elucidate the chemical identity or structure of molecules and other chemical compounds. In a typical MS procedure, a sample, which may be solid, liquid, or gaseous, is ionized, for example by bombarding it with a beam of electrons. This may cause some of the sample's molecules to break up into positively charged fragments or simply become positively charged without fragmenting. These ions (fragments) are then separated accor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Optical Spectrometer
An optical spectrometer (spectrophotometer, spectrograph or spectroscope) is an instrument used to measure properties of light over a specific portion of the electromagnetic spectrum, typically used in spectroscopic analysis to identify materials. The variable measured is most often the irradiance of the light but could also, for instance, be the polarization state. The independent variable is usually the wavelength of the light or a closely derived physical quantity, such as the corresponding wavenumber or the photon energy, in units of measurement such as centimeters, reciprocal centimeters, or electron volts, respectively. A spectrometer is used in spectroscopy for producing spectral lines and measuring their wavelengths and intensities. Spectrometers may operate over a wide range of non-optical wavelengths, from gamma rays and X-rays into the far infrared. If the instrument is designed to measure the spectrum on an absolute scale rather than a relative one, then it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
History Of Spectroscopy
Modern spectroscopy in the Western world started in the 17th century. New designs in optics, specifically prisms, enabled systematic observations of the solar spectrum. Isaac Newton first applied the word ''spectrum'' to describe the rainbow of colors that combine to form white light. During the early 1800s, Joseph von Fraunhofer conducted experiments with dispersive spectrometers that enabled spectroscopy to become a more precise and quantitative scientific technique. Since then, spectroscopy has played and continues to play a significant role in chemistry, physics and astronomy. Fraunhofer observed and measured dark lines in the Sun's spectrum, which now bear his name although several of them were observed earlier by Wollaston. Origins and experimental development The Romans were already familiar with the ability of a prism to generate a rainbow of colors. Newton is traditionally regarded as the founder of spectroscopy, but he was not the first scientist who studied and report ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectroscopy
Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum. Spectroscopy, primarily in the electromagnetic spectrum, is a fundamental exploratory tool in the fields of astronomy, chemistry, materials science, and physics, allowing the composition, physical structure and electronic structure of matter to be investigated at the atomic, molecular and macro scale, and over astronomical distances. Historically, spectroscopy originated as the study of the wavelength dependence of the absorption by gas phase matter of visible light dispersed by a prism. Current applications of spectroscopy include biomedical spectroscopy in the areas of tissue analysis and medical imaging. Matter waves and acoustic waves can also be considered forms of radiative energy, and recently gravitational waves have been associa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atoms
Atoms are the basic particles of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other by the number of protons that are in their atoms. For example, any atom that contains 11 protons is sodium, and any atom that contains 29 protons is copper. Atoms with the same number of protons but a different number of neutrons are called isotopes of the same element. Atoms are extremely small, typically around 100 picometers across. A human hair is about a million carbon atoms wide. Atoms are smaller than the shortest wavelength of visible light, which means humans cannot see atoms with conventional microscopes. They are so small that accurately predicting their behavior using classical physics is not possible due to quantum effects. More than 99.94% of an atom's mass is in the nucleus. Protons have a positive electric charge a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Absorption Spectrum
Absorption spectroscopy is spectroscopy that involves techniques that measure the absorption of electromagnetic radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a function of frequency, and this variation is the absorption spectrum. Absorption spectroscopy is performed across the electromagnetic spectrum. Absorption spectroscopy is employed as an analytical chemistry tool to determine the presence of a particular substance in a sample and, in many cases, to quantify the amount of the substance present. Infrared and ultraviolet–visible spectroscopy are particularly common in analytical applications. Absorption spectroscopy is also employed in studies of molecular and atomic physics, astronomical spectroscopy and remote sensing. There is a wide range of experimental approaches for measuring absorption spectra. The most common ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analytical Chemistry
Analytical skill, Analytical chemistry studies and uses instruments and methods to Separation process, separate, identify, and Quantification (science), quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analytes. Qualitative inorganic analysis, Qualitative analysis identifies analytes, while Quantitative analysis (chemistry), quantitative analysis determines the numerical amount or concentration. Analytical chemistry consists of classical, wet chemistry, wet chemical methods and modern analytical techniques. Classical qualitative methods use separations such as Precipitation (chemistry), precipitation, Extraction (chemistry), extraction, and distillation. Identification may be based on differences in color, odor, melting point, boiling point, solubility, radioactivity or reactivity. Classical quantitative analysis uses mass or volume changes to quantify amount. Ins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Continuous Spectrum
In the physical sciences, the term ''spectrum'' was introduced first into optics by Isaac Newton in the 17th century, referring to the range of colors observed when white light was dispersion (optics), dispersed through a prism (optics), prism. Soon the term referred to a plot of light intensity (physics), intensity or power (physics), power as a function of frequency or wavelength, also known as a ''spectral density plot''. Later it expanded to apply to other waves, such as sound waves and sea waves that could also be measured as a function of frequency (e.g., noise spectrum, sea wave spectrum). It has also been expanded to more abstract "signals", whose power spectrum can be spectrum analyzer, analyzed and signal processing, processed. The term now applies to any signal that can be measured or decomposed along a continuous variable, such as energy in electron spectroscopy or mass-to-charge ratio in mass spectrometry. Spectrum is also used to refer to a graphical representation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Astronomical Spectroscopy
Astronomical spectroscopy is the study of astronomy using the techniques of spectroscopy to measure the electromagnetic spectrum, spectrum of electromagnetic radiation, including Visible light astronomy, visible light, Ultraviolet astronomy, ultraviolet, X-ray astronomy, X-ray, Infrared astronomy, infrared and Radio astronomy, radio waves that radiant energy, radiate from stars and other celestial objects. A stellar spectrum can reveal many properties of stars, such as their chemical composition, temperature, density, mass, distance and luminosity. Spectroscopy can show the velocity of motion towards or away from the observer by measuring the Doppler effect, Doppler shift. Spectroscopy is also used to study the physical properties of many other types of celestial objects such as planets, nebulae, Galaxy, galaxies, and Active galactic nucleus, active galactic nuclei. Background Astronomical spectroscopy is used to measure three major bands of radiation in the electromagnetic spe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diffraction Grating
In optics, a diffraction grating is an optical grating with a periodic structure that diffraction, diffracts light, or another type of electromagnetic radiation, into several beams traveling in different directions (i.e., different diffraction angles). The emerging coloration is a form of structural coloration. The directions or diffraction angles of these beams depend on the wave (light) Angle of incidence (optics), incident angle to the diffraction grating, the spacing or periodic distance between adjacent diffracting elements (e.g., parallel slits for a transmission grating) on the grating, and the wavelength of the incident light. The grating acts as a dispersion (optics), dispersive element. Because of this, diffraction gratings are commonly used in monochromators and spectrometers, but other applications are also possible such as optical encoders for high-precision motion control and wavefront measurement. For typical applications, a reflection (optics), reflective grati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectrum
A spectrum (: spectra or spectrums) is a set of related ideas, objects, or properties whose features overlap such that they blend to form a continuum. The word ''spectrum'' was first used scientifically in optics to describe the rainbow of colors in visible light after passing through a prism. In the optical spectrum, light wavelength is viewed as continuous, and spectral colors are seen to blend into one another smoothly when organized in order of their corresponding wavelengths. As scientific understanding of light advanced, the term came to apply to the entire electromagnetic spectrum, including radiation not visible to the human eye. ''Spectrum'' has since been applied by analogy to topics outside optics. Thus, one might talk about the " spectrum of political opinion", or the "spectrum of activity" of a drug, or the " autism spectrum". In these uses, values within a spectrum may not be associated with precisely quantifiable numbers or definitions. Such uses imply a bro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |