|
Self-diffusion
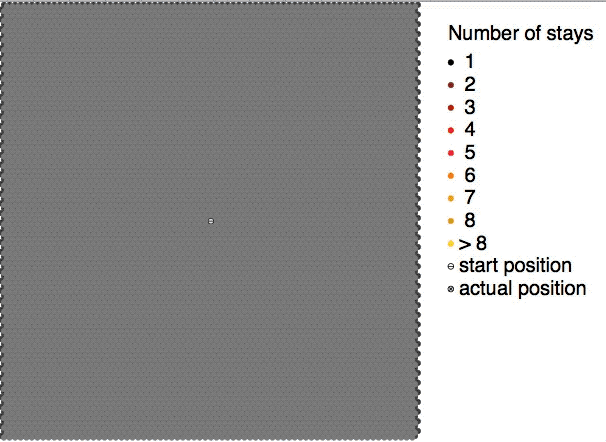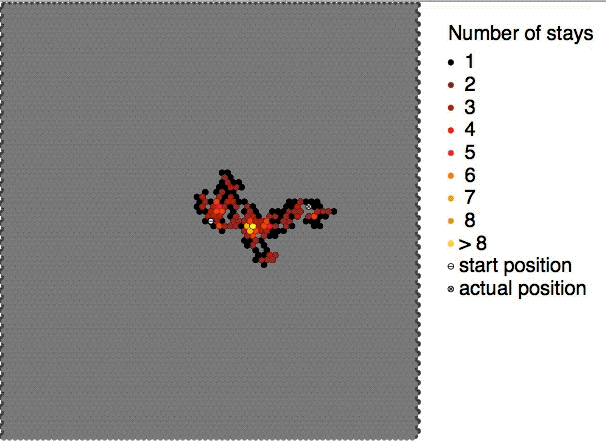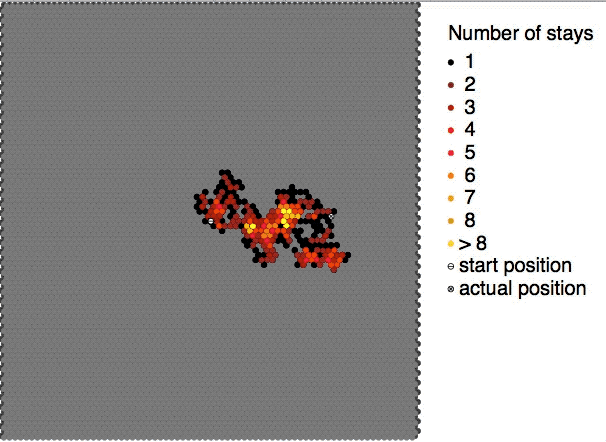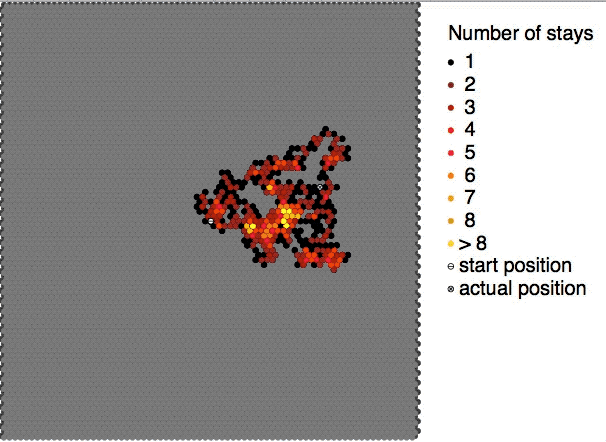
Self-diffusion describes the diffusive motions of molecules within themselves e.g. the movement of a water molecule in water. According to the IUPAC definition, the self-diffusion coefficient D_i^* of medium i is the diffusion coefficient D_i of a chemical species in said medium when the concentration of this species is extrapolated to zero concentration. It can be described by the equation: D_i^* = D_i\frac Here, a_i is the activity of the medium i in the solution and c_i is the concentration of medium i. Due to challenges observing it directly it is commonly assumed to be equal to the diffusion of an isotope in the medium of interest. However modern simulations are able to estimate it directly without the need for isotope labeling. See also * Brownian motion * Diffusion * Molecular diffusion Molecular diffusion is the motion of atoms, molecules, or other particles of a gas or liquid at temperatures above absolute zero. The rate of this movement is a function of tem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Diffusion
Molecular diffusion is the motion of atoms, molecules, or other particles of a gas or liquid at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid, size and density (or their product, mass) of the particles. This type of diffusion explains the net flux of molecules from a region of higher concentration to one of lower concentration. Once the concentrations are equal the molecules continue to move, but since there is no concentration gradient the process of molecular diffusion has ceased and is instead governed by the process of self-diffusion, originating from the random motion of the molecules. The result of diffusion is a gradual mixing of material such that the distribution of molecules is uniform. Since the molecules are still in motion, but an equilibrium has been established, the result of molecular diffusion is called a "dynamic equilibrium". In a Phase (matter), phase with uniform temperature, absent external n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical potential. It is possible to diffuse "uphill" from a region of lower concentration to a region of higher concentration, as in spinodal decomposition. Diffusion is a stochastic process due to the inherent randomness of the diffusing entity and can be used to model many real-life stochastic scenarios. Therefore, diffusion and the corresponding mathematical models are used in several fields beyond physics, such as statistics, probability theory, information theory, neural networks, finance, and marketing. The concept of diffusion is widely used in many fields, including physics (Molecular diffusion, particle diffusion), chemistry, biology, sociology, economics, statistics, data science, and finance (diffusion of people, ideas, data and price v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Diffusivity
Diffusivity, mass diffusivity or diffusion coefficient is usually written as the proportionality constant between the molar flux due to molecular diffusion and the negative value of the gradient in the concentration of the species. More accurately, the diffusion coefficient times the local concentration is the proportionality constant between the negative value of the mole fraction gradient and the molar flux. This distinction is especially significant in gaseous systems with strong temperature gradients. Diffusivity derives its definition from Fick's law and plays a role in numerous other equations of physical chemistry. The diffusivity is generally prescribed for a given pair of species and pairwise for a multi-species system. The higher the diffusivity (of one substance with respect to another), the faster they diffuse into each other. Typically, a compound's diffusion coefficient is ~10,000× as great in air as in water. Carbon dioxide in air has a diffusion coefficient of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. IUPAC's executive director heads this administrative office, currently Greta Heydenrych. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national List of chemistry societies, chemistry societies, national Academy of Sciences, academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blackwell Scientific Publications
Wiley-Blackwell is an international scientific, technical, medical, and scholarly publishing business of John Wiley & Sons. It was formed by the merger of John Wiley & Sons Global Scientific, Technical, and Medical business with Blackwell Publishing in 2007. Wiley-Blackwell is now an imprint that publishes a diverse range of academic and professional fields, including biology, medicine, physical sciences, technology, social science, and the humanities. Blackwell Publishing history Blackwell Publishing was formed by the 2001 merger of two Oxford-based academic publishing companies, Blackwell Science, founded in 1939 as Blackwell Scientific Publishing, and Blackwell Publishers, founded in 1922 as Basil Blackwell & Mott. Blackwell Publishers, founded in 1926, had its origins in the 19th century Blackwell's family bookshop and publishing business. The merger between the two publishing companies created the world's leading learned society publisher. The group then acquired BMJ Books ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Species
Chemical species are a specific form of chemical substance or chemically identical molecular entities that have the same molecular energy level at a specified timescale. These entities are classified through bonding types and relative abundance of isotopes. Types of chemical species can be classified based on the type of molecular entity and can be either an atomic, molecular, ionic or radical species. Classification Generally, a chemical species is defined as a chemical identity that has the same set of molecular energy levels in a defined timescale (i.e. an experiment). These energy levels determine the way the chemical species will interact with others through properties such as bonding or isotopic compositions. The chemical species can be an atom, molecule, ion, or radical, with a specific chemical name and chemical formula. In supramolecular chemistry, chemical species are structures created by forming or breaking bonds between molecules, such as hydrogen bonding, dipole ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activity (chemistry)
In thermodynamics, activity (symbol ) is a measure of the "effective concentration" of a species in a mixture, in the sense that the species' chemical potential depends on the activity of a real solution in the same way that it would depend on concentration for an ideal solution. The term "activity" in this sense was coined by the American chemist Gilbert N. Lewis in 1907. By convention, activity is treated as a dimensionless quantity, although its value depends on customary choices of standard state for the species. The activity of pure substances in condensed phases (solids and liquids) is taken as = 1. Activity depends on temperature, pressure and composition of the mixture, among other things. For gases, the activity is the effective partial pressure, and is usually referred to as fugacity. The difference between activity and other measures of concentration arises because the interactions between different types of molecules in non-ideal gases or solutions are differen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solution (chemistry)
In chemistry, a solution is defined by IUPAC as "A liquid or solid phase containing more than one substance, when for convenience one (or more) substance, which is called the solvent, is treated differently from the other substances, which are called solutes. When, as is often but not necessarily the case, the sum of the mole fractions of solutes is small compared with unity, the solution is called a dilute solution. A superscript attached to the ∞ symbol for a property of a solution denotes the property in the limit of infinite dilution." One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term " aqueous solution" is used when one of the solvents is water. Types ''Homogeneous'' means that the components of the mixture form a single phase. ''Heterogeneous'' means that the components of the mixture are of different phase. The properties of the mixture (such as concentration, temp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', '' molar concentration'', '' number concentration'', and '' volume concentration''. The concentration can refer to any kind of chemical mixture, but most frequently refers to solutes and solvents in solutions. The molar (amount) concentration has variants, such as normal concentration and osmotic concentration. Dilution is reduction of concentration, e.g. by adding solvent to a solution. The verb to concentrate means to increase concentration, the opposite of dilute. Etymology ''Concentration-'', ''concentratio'', action or an act of coming together at a single place, bringing to a common center, was used in post-classical Latin in 1550 or earlier, similar terms attested in Italian (1589), Spanish (1589), English (1606), French (1632). Qualitative description Often in informal, non- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopic Labeling
Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope (an atom with a detectable variation in neutron count) through chemical reaction, metabolic pathway, or a biological cell. The reactant is 'labeled' by replacing one or more specific atoms with their isotopes. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine what sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling. In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes; through their mass, vibrational mode, or radioactive decay. Mass spectrometry detects the difference in an isotope's mass, while infrared spectroscopy detects the difference in the isotope's vibrational modes. Nuclear magnetic resonance ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brownian Motion
Brownian motion is the random motion of particles suspended in a medium (a liquid or a gas). The traditional mathematical formulation of Brownian motion is that of the Wiener process, which is often called Brownian motion, even in mathematical sources. This motion pattern typically consists of Randomness, random fluctuations in a particle's position inside a fluid sub-domain, followed by a relocation to another sub-domain. Each relocation is followed by more fluctuations within the new closed volume. This pattern describes a fluid at thermal equilibrium, defined by a given temperature. Within such a fluid, there exists no preferential direction of flow (as in transport phenomena). More specifically, the fluid's overall Linear momentum, linear and Angular momentum, angular momenta remain null over time. The Kinetic energy, kinetic energies of the molecular Brownian motions, together with those of molecular rotations and vibrations, sum up to the caloric component of a fluid's in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |