|
Reactive Nitrogen Species
Reactive nitrogen species (RNS) are a family of antimicrobial molecules derived from nitric oxide (•NO) and superoxide (O2•âˆ’) produced via the enzymatic activity of inducible nitric oxide synthase 2 ( NOS2) and NADPH oxidase respectively. NOS2 is expressed primarily in macrophages after induction by cytokines and microbial products, notably interferon-gamma (IFN-γ) and lipopolysaccharide (LPS). Reactive nitrogen species act together with reactive oxygen species (ROS) to damage cells, causing nitrosative stress. Therefore, these two species are often collectively referred to as ROS/RNS. Reactive nitrogen species are also continuously produced in plants as by-products of aerobic metabolism or in response to stress. Types RNS are produced in animals starting with the reaction of nitric oxide (•NO) with superoxide (O2•âˆ’) to form peroxynitrite (ONOO−): * •NO (nitric oxide) + O2•âˆ’ (superoxide) → ONOO− (peroxynitrite) Superoxide anion (O2â ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ... (•N=O or •NO). Nitric oxide is also a heteronuclear diatomic molecule, a class of molecules whose study spawned early modern molecular orbital theory, theories of chemical bonding. An important Reaction intermediate, intermediate in chemical industry, industrial chemistry, nitric oxide forms in combustion systems and can be generated by lightning in thunderstorms. In mammals, including humans, nitric oxide is a signaling molecule in many physiological and pathological pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physiological Reviews
''Physiological Reviews'' is a journal published quarterly by the American Physiological Society which has been published since 1921. The editor in chief of the journal is Sadis Matalon (University of Alabama at Birmingham). The journal's first managing editor, who served to his death in 1946, was Dr. Donald R. Hooker. Among the cadre of editors at the journal's inception were William Henry Howell, Lafayette Mendel, and John Macleod. From 1932 to 1950, the chairman of the board of editors of the journal was Anton J. Carlson. Other notable people who have served on the journal's editorial board include John Jacob Abel (c.1935), Ernest William Goodpasture (c.1938). According to the Journal Citation Reports, the journal has a 2020 impact factor of 37.312Internet Archive page set(values in table linked to specific pages) References Further reading * ResearchGate ResearchGate is a European commercial social networking site for scientists and researchers to share papers, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactive Carbonyl Species
Reactive carbonyl species (RCS) are molecules with highly reactive carbonyl groups, and often known for their damaging effects on proteins, nucleic acids, and lipids. They are often generated as metabolic products. Important RCSs include 3-deoxyglucosone, glyoxal, and methylglyoxal. RCSs react with amines and thiol groups leading to advanced glycation endproducts (AGEs). AGE's are indicators of diabetes. Reactive aldehyde species (RASP), such as malondialdehyde and 4-hydroxynonenal, are a subset of RCS that are implicated in a variety of human diseases. See also * Reactive oxygen species * Reactive sulfur species * Reactive nitrogen species Reactive nitrogen species (RNS) are a family of antimicrobial molecules derived from nitric oxide (•NO) and superoxide (O2•âˆ’) produced via the enzymatic activity of inducible nitric oxide synthase 2 ( NOS2) and NADPH oxidase respectivel ... References Molecules Carbon compounds {{Biochemistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactive Sulfur Species
Reactive sulfur species (RSS) are a family of sulfur-based chemical compounds that can oxidize and inhibit thiol-proteins and enzymes. They are often formed by the oxidation of thiols and disulfides into higher oxidation states. Examples of RSS include persulfides, polysulfides and thiosulfate. See also * Reactive oxygen species * Reactive nitrogen species Reactive nitrogen species (RNS) are a family of antimicrobial molecules derived from nitric oxide (•NO) and superoxide (O2•âˆ’) produced via the enzymatic activity of inducible nitric oxide synthase 2 ( NOS2) and NADPH oxidase respectivel ... * Reactive carbonyl species References {{Reflist Molecules Sulfur compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactive Oxygen Species
In chemistry, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen (). Examples of ROS include peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha-oxygen. The reduction of molecular oxygen () produces superoxide (), which is the precursor to most other reactive oxygen species: :O2 + e^- -> \ ^\bullet O2- Dismutation of superoxide produces hydrogen peroxide (): :2 H+ + \ ^\bullet O2^- + \ ^\bullet O2^- -> H2O2 + O2 Hydrogen peroxide in turn may be partially reduced, thus forming hydroxide ions and hydroxyl radicals (), or fully reduced to water: :H2O2 + e^- -> HO^- + \ ^\bullet OH :2 H+ + 2 e- + H2O2 -> 2 H2O In a biological context, ROS are byproducts of the normal metabolism of oxygen. ROS have roles in cell signaling and homeostasis. ROS are intrinsic to cellular functioning, and are present at low and stationary levels in normal cells. In plants, ROS are involved in metabolic processes related to photoprotection and toleran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome C
The cytochrome complex, or cyt ''c'', is a small hemeprotein found loosely associated with the inner membrane of the mitochondrion. It belongs to the cytochrome c family of proteins and plays a major role in cell apoptosis. Cytochrome c is highly water-soluble, unlike other cytochromes, and is an essential component of the respiratory electron transport chain, where it carries one electron. It is capable of undergoing oxidation and reduction as its iron atom converts between the ferrous and ferric forms, but does not bind oxygen. It transfers electrons between Complexes III (Coenzyme Q – Cyt c reductase) and IV (Cyt c oxidase). In humans, cytochrome c is encoded by the ''CYCS'' gene. Species distribution Cytochrome c is a highly conserved protein across the spectrum of eukaryotic species, found in plants, animals, fungi, and many unicellular organisms. This, along with its small size (molecular weight about 12,000 daltons), makes it useful in studies of cladistics. Cyt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. It is a trace gas in Earth's atmosphere at 421 parts per million (ppm), or about 0.04% by volume (as of May 2022), having risen from pre-industrial levels of 280 ppm. Burning fossil fuels is the primary cause of these increased CO2 concentrations and also the primary cause of climate change.IPCC (2022Summary for policy makersiClimate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
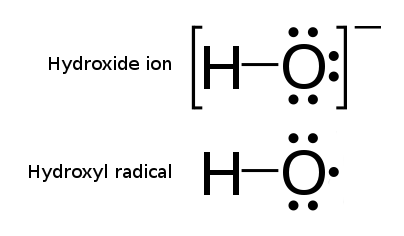
Hydroxyl Radical
The hydroxyl radical is the diatomic molecule . The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals are produced from the decomposition of hydroperoxides (ROOH) or, in atmospheric chemistry, by the reaction of excited atomic oxygen with water. It is also important in the field of radiation chemistry, since it leads to the formation of hydrogen peroxide and oxygen, which can enhance corrosion and SCC in coolant systems subjected to radioactive environments. In organic synthesis, hydroxyl radicals are most commonly generated by photolysis of 1-hydroxy-2(1''H'')-pyridinethione. Notation The unpaired electron of the hydroxyl radical is officially represented by a middle dot, •, beside the O. Biology Hydroxyl radicals can occasionally be produced as a byproduct of immune action. Macrophages and microglia most frequently generate this compound when exp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxynitrous Acid
Peroxynitrous acid (HNO3) is a reactive nitrogen species (RNS). It is the conjugate acid of peroxynitrite (ONOO−). It has a p''K''a of approximately 6.8. It is formed ''in vivo'' from the diffusion-controlled reaction of nitrogen monoxide (ON•) and superoxide (). It is an isomer of nitric acid and isomerises with a rate constant of ''k'' = 1.2 s−1, a process whereby up to 5% of hydroxyl and nitrogen dioxide radicals may be formed. It oxidises and nitrates aromatic compounds in low yield. The mechanism may involve a complex between the aromatic compound and ONOOH, and a transition from the ''cis''- to the ''trans''-configuration of ONOOH. Peroxynitrous acid is also important in atmospheric chemistry Atmospheric chemistry is a branch of atmospheric science in which the chemistry of the Earth's atmosphere and that of other planets is studied. It is a multidisciplinary approach of research and draws on environmental chemistry, physics, meteorol .... References ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radical (chemistry)
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes. A notable example of a radical is the hydroxyl radical (HO·), a molecule that has one unpaired electron on the oxygen atom. Two other examples are triplet oxygen and triplet carbene (꞉) which have two unpaired electrons. Radicals may be generated in a number of ways, but typical methods involve redox reactions. Ionizing radiation, heat, electrical discharges, and electrolysis are known to produce radicals. Radicals are intermediates in many chemical reactions, more so than is apparent from the balanced equations. Radicals are important in combustion, atmospheric chemistry, polymerization, plasma chemistry, biochemistry, and many other chemical processes. A majority of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
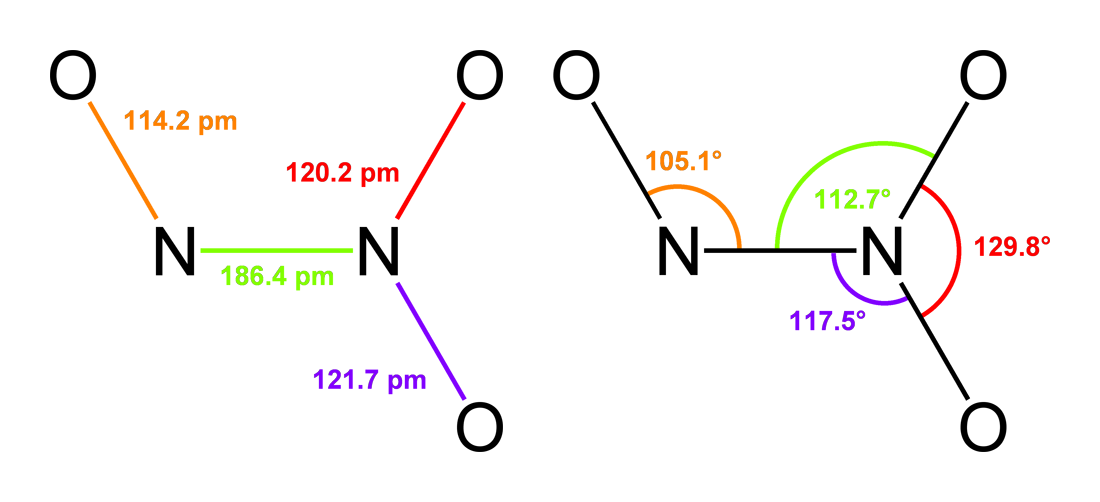
Dinitrogen Trioxide
Dinitrogen trioxide is the chemical compound with the formula N2O3. It is one of the simple nitrogen oxides. It forms upon mixing equal parts of nitric oxide and nitrogen dioxide and cooling the mixture below −21 °C (−6 °F): :NO + NO2 N2O3 Dinitrogen trioxide is only isolable at low temperatures, i.e. in the liquid and solid phases. In liquid and solid states, it has a deep blue color. At higher temperatures the equilibrium favors the constituent gases, with ''K''diss = 193 kPa (25 °C). This compound is sometimes called "nitrogen trioxide", but this name properly refers to another compound, the (uncharged) nitrate radical . Structure and bonding Typically, N–N bonds are similar in length to that in hydrazine (145 pm). Dinitrogen trioxide, however, has an unusually long N–N bond at 186 pm. Some other nitrogen oxides also possess long N–N bonds, including dinitrogen tetroxide (175 pm). The N2O3 molecule is planar and exhibits Cs symmet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Dioxide
Nitrogen dioxide is a chemical compound with the formula . It is one of several nitrogen oxides. is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year for use primarily in the production of fertilizers. At higher temperatures it is a reddish-brown gas. It can be fatal if inhaled in large quantities. Nitrogen dioxide is a paramagnetic, bent molecule with C2v point group symmetry. It is included in the NOx family of atmospheric pollutants. Properties Nitrogen dioxide is a reddish-brown gas with a pungent, acrid odor above , becomes a yellowish-brown liquid below , and converts to the colorless dinitrogen tetroxide () below . The bond length between the nitrogen atom and the oxygen atom is 119.7 pm. This bond length is consistent with a bond order between one and two. Unlike ozone, O3, the ground electronic state of nitrogen dioxide is a doublet state, since nitrogen has one unpaired electron, which decreases ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |