|
Rucaparib
Rucaparib, sold under the brand name Rubraca, is a PARP inhibitor used as an anti-cancer agent. Rucaparib is a first-in-class pharmaceutical drug targeting the DNA repair enzyme poly-ADP ribose polymerase-1 (PARP-1). It is taken by mouth. The most common side effects include tiredness or weakness, nausea (feeling sick), increased levels of creatinine (which may indicate kidney problems) and liver enzymes in the blood (which may indicate liver damage), vomiting, anaemia (low red blood cell counts), decreased appetite, dysgeusia (taste disturbances), diarrhoea, thrombocytopenia (low levels of platelets) and abdominal pain (belly ache). Medical uses Rucaparib is indicated as monotherapy for the maintenance treatment of adults with platinum-sensitive relapsed high-grade epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in response (complete or partial) to platinum-based chemotherapy. In the United States, rucaparib is also indicated for the treatment of pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clovis Oncology
Clovis Oncology is an American pharmaceutical company which mainly markets products for treatment in oncology. Clovis was founded in 2009 and is headquartered in Boulder, Colorado. The company is a publicly traded company on NASDAQ under the symbol CLVS and is in the NASDAQ Biotechnology Index with several products in its product pipeline. As of December 31, 2017, the company was not profitable and had incurred losses in each year since its inception in April 2009. In December 2022, Clovis Oncology filed for Chapter 11 bankruptcy. History Clovis Oncology was founded in 2009 by Patrick Mahaffy in Boulder, Colorado. The company was named in honor of the Mahaffy cache, a collection of Clovis culture, Clovis period stone tools dated to 11,000 BCE, discovered in the front yard of Mahaffy's home in Boulder. Product development Rociletinib The company was developing rociletinib, as a treatment for non-small cell lung cancer. A phase III trial was completed in April 2016 and had ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cancer Treatments
Cancer treatments are a wide range of treatments available for the many different types of cancer, with each cancer type needing its own specific treatment. Treatments can include surgery, chemotherapy, radiation therapy, hormonal therapy, targeted therapy including small-molecule drugs or monoclonal antibodies, and PARP inhibitors such as olaparib. Other therapies include hyperthermia, immunotherapy, photodynamic therapy, and stem-cell therapy. Most commonly cancer treatment involves a series of separate therapies such as chemotherapy before surgery. Angiogenesis inhibitors are sometimes used to enhance the effects of immunotherapies. The choice of therapy depends upon the location and grade of the tumor and the stage of the disease, as well as the general state of the patient. Biomarker testing can help to determine the type of cancer, and indicate the best therapy. A number of experimental cancer treatments are continuously under development. In 2023 it was estimated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Accelerated Approval
The United States Food and Drug Administration (FDA) initiated the FDA Accelerated Approval Program in 1992 to allow faster approval of drugs for serious conditions that fill an unmet medical need. The faster approval relies on use of surrogate endpoints. Drug approval typically requires clinical trials with endpoints that demonstrate a clinical benefit, such as increased survival for cancer patients. Drugs with accelerated approval can initially be tested in clinical trials that use a surrogate endpoint, or something that is thought to predict clinical benefit. Surrogate endpoints typically require less time, and in the case of a cancer patient, it is much faster to measure a reduction in tumor size, for example, than overall patient survival. Drugs approved under the FDA Accelerated Approval Program still need to be tested in clinical trials using endpoints that demonstrate clinical benefit, and those trials are known as phase 4 confirmatory trials. If the drug later proves unabl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PARP Inhibitors
PARP inhibitors are a class of drugs that are a group of pharmacological inhibitors of the enzyme poly ADP ribose polymerase (PARP), which plays a role in repairing DNA in damaged cells. Medical uses of these drugs include the treatment of heritable cancers. Several forms of cancer are more dependent on PARP than regular cells, making PARP (PARP1, PARP2 etc.) an attractive target for cancer therapy. PARP inhibitors appear to improve progression-free survival in women with recurrent platinum-sensitive ovarian cancer, as evidenced mainly by olaparib added to conventional treatment. In addition to their use in cancer therapy, PARP inhibitors are considered a potential treatment for acute life-threatening diseases, such as stroke and myocardial infarction, as well as for long-term neurodegenerative diseases. Medical uses Approved for marketing * Olaparib: In December, 2014, the EMA and US FDA approved olaparib as monotherapy (at 400 mg taken twice per day) for patients ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactams
A lactam is a cyclic amide, formally derived from an amino alkanoic acid through cyclization reactions. The term is a portmanteau of the words '' lactone'' + ''amide''. Nomenclature Greek prefixes in alphabetical order indicate ring size. This ring-size nomenclature stems from the fact that hydrolysis of an α-lactam gives an α-amino acid and that of a β-Lactam gives a β-amino acid, and so on. Synthesis General synthetic methods are used for the organic synthesis of lactams. Beckmann rearrangement Lactams form by the acid-catalyzed rearrangement of oximes in the Beckmann rearrangement. Schmidt reaction Lactams form from cyclic ketones and hydrazoic acid in the Schmidt reaction. Cyclohexanone with hydrazoic acid, forms ε - Caprolactum, which upon treatment with excess acid forms Cardiazole, a heart stimulant. Cyclization of amino acids Lactams can be formed from cyclisation of amino acids via the coupling between an amine and a carboxylic acid within the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP2D6 Inhibitors
Cytochrome P450 2D6 (CYP2D6) is an enzyme that in humans is encoded by the ''CYP2D6'' gene. ''CYP2D6'' is primarily expressed in the liver. It is also highly expressed in areas of the central nervous system, including the substantia nigra. CYP2D6, a member of the cytochrome P450 mixed-function oxidase system, is one of the most important enzymes involved in the metabolism of xenobiotics in the body. In particular, CYP2D6 is responsible for the metabolism and elimination of approximately 25% of clinically used drugs, via the addition or removal of certain functional groups – specifically, hydroxylation, demethylation, and dealkylation. CYP2D6 also activates some prodrugs. This enzyme also metabolizes several endogenous substances, such as N,N-Dimethyltryptamine, hydroxytryptamines, neurosteroids, and both ''m''-tyramine and ''p''-tyramine which CYP2D6 metabolizes into dopamine in the brain and liver. Considerable variation exists in the efficiency and amount of C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzazepines
Benzazepines are heterocyclic chemical compounds consisting of a benzene ring fused to an azepine ring. Examples include: File:Benazepril structure.svg, Benazepril File:Fenoldopam.svg, Fenoldopam File:GSK-189,254.svg, GSK-189,254 File:Ivabradine 2.svg, Ivabradine File:Semagacestat structure.svg, Semagacestat File:Varenicline.svg, Varenicline File:Trepipam.svg, Trepipam File:SKF38393.svg, SKF-38,393 See also * Benzodiazepine Benzodiazepines (BZD, BDZ, BZs), colloquially known as "benzos", are a class of central nervous system (CNS) depressant, depressant drugs whose core chemical structure is the fusion of a benzene ring and a diazepine ring. They are prescribed t ... * Dibenzazepine References {{Heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Committee For Medicinal Products For Human Use
The Committee for Medicinal Products for Human Use (CHMP), formerly known as the Committee for Proprietary Medicinal Products (CPMP), is the European Medicines Agency's committee responsible for elaborating the agency's opinions on all issues regarding medicinal product Medication (also called medicament, medicine, pharmaceutical drug, medicinal product, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy ( pharmacotherapy) is an important part of the ...s for human use. See also * Committee for Medicinal Products for Veterinary Use References External links Committee for Medicinal Products for Human Use (CHMP) Health and the European Union {{eu-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of pharmaceutical products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orphan Drug
An orphan drug is a medication, pharmaceutical agent that is developed to treat certain rare medical conditions. An orphan drug would not be profitable to produce without government assistance, due to the small population of patients affected by the conditions. The conditions that orphan drugs are used to treat are referred to as orphan diseases. The assignment of orphan status to a disease and to drugs developed to treat it is a matter of public policy that depends on the legislation (if there is any) of the country. Designation of a drug as an orphan drug has yielded medical breakthroughs that might not otherwise have been achieved, due to the economics of drug medical research, research and development. Examples of this can be that in the U.S. and the EU, it is easier to gain marketing approval for an orphan drug. There may be other financial incentives, such as an extended period of exclusivity, during which the producer has sole rights to market the drug. All are intended to en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
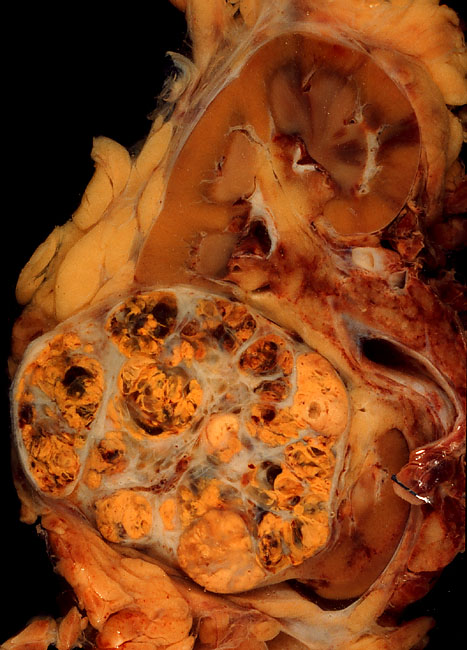
Ovarian Cancer
Ovarian cancer is a cancerous tumor of an ovary. It may originate from the ovary itself or more commonly from communicating nearby structures such as fallopian tubes or the inner lining of the abdomen. The ovary is made up of three different cell types including epithelial cells, germ cells, and stromal cells. When these cells become abnormal, they have the ability to divide and form tumors. These cells can also invade or spread to other parts of the body. When this process begins, there may be no or only vague symptoms. Symptoms become more noticeable as the cancer progresses. These symptoms may include bloating, vaginal bleeding, pelvic pain, abdominal swelling, constipation, and loss of appetite, among others. Common areas to which the cancer may spread include the lining of the abdomen, lymph nodes, lungs, and liver. The risk of ovarian cancer increases with age. Most cases of ovarian cancer develop after menopause. It is also more common in women who have ovulated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |