|
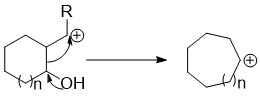
Ring Contraction
Ring expansion and ring contraction reactions expand or contract Ring (chemistry), rings, usually in organic chemistry. The term usually refers to reactions involve making and breaking C-C bonds, Diverse pathways lead to these kinds of reactions. Many of these reactions are primarily of theoretical or pedagoogical interest, but some are very useful. Ring expansions Rings can be expanded by attack of the ring onto an outside group already appended to the ring (a Migration (chemistry), migration/insertion), opening of a bicycle to a single larger ring, or coupling a ring closing with an expansion. These expansions can be further broken down by what type of atom they incorporate (a carbon or a heteroatom) into the expanded ring. Carbon insertion through migration to an exocyclic group These reactions have the general features of having an exocyclic leaving group on a carbon adjacent to the ring and an electron donating group on the ring capable of initiating a migration of an en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ring (chemistry)
In chemistry, a ring is an ambiguous term referring either to a simple cycle of atoms and bonds in a molecule or to a connected set of atoms and bonds in which every atom and bond is a member of a cycle (also called a ring system). A ring system that is a simple cycle is called a monocycle or simple ring, and one that is not a simple cycle is called a polycycle or polycyclic ring system. A simple ring contains the same number of sigma bonds as atoms, and a polycyclic ring system contains more sigma bonds than atoms. A molecule containing one or more rings is called a cyclic compound, and a molecule containing two or more rings (either in the same or different ring systems) is termed a polycyclic compound. A molecule containing no rings is called an acyclic or open-chain compound. Homocyclic and heterocyclic rings A homocycle or homocyclic ring is a ring in which all atoms are of the same chemical element. A heterocycle or heterocyclic ring is a ring containing atoms of at least ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocycle
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of organic heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of organic heterocyclic chemistry focuses especially on organic unsaturated derivatives, and the preponderance of work and applications involves unstrained organic 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of organic heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Original Baeyer-Villiger Oxidation Reactions (cropped)
Originality is the aspect of created or invented works that distinguish them from reproductions, clones, forgeries, or substantially derivative works. The modern idea of originality is according to some scholars tied to Romanticism, by a notion that is often called romantic originality.Smith (1924)Waterhouse (1926)Macfarlane (2007) The validity of "originality" as an operational concept has been questioned. For example, there is no clear boundary between "derivative" and "inspired by" or "in the tradition of." The concept of originality is both culturally and historically contingent. For example, unattributed reiteration of a published text in one culture might be considered plagiarism but in another culture might be regarded as a convention of veneration. At the time of Shakespeare, it was more common to appreciate the similarity with an admired classical work, and Shakespeare himself avoided "unnecessary invention".Royal Shakespeare Company (2007) ''The RSC Shakespeare - Will ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schmidt Ring Expansion
Schmidt may refer to: * Schmidt (surname), including list of people and fictional characters with the surname * Schmidt (singer) (born 1990), German pop and jazz singer * Schmidt (lunar crater), a small lunar impact crater * Schmidt (Martian crater), a crater on Mars * Schmidt (volcano), in Kamchatka * Schmidt Block, listed on the National Register of Historic Places in Scott County, Iowa, USA * Schmidt Brewery, a St. Paul brewery * Schmidt camera, an astronomical telescope designed for photography * Schmidt–Cassegrain telescope, a version of the Schmidt camera * Schmidt Site, an archeological site in Michigan, USA, listed on the National Register of Historic Places in 1973 * Schmidt Spiele, a German games manufacturer * Schmidt Baking Company, makers of Schmidt's Blue Ribbon Bread * von Schmidt auf Altenstadt, a German baronial family in Kirchgattendorf, part of the municipality of Gattendorf * Schmidt Island, an island in the Novaya Zemlya archipelago in the Arctic Ocean * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schmidt Reaction
In organic chemistry, the Schmidt reaction is an organic reaction in which an azide reacts with a carbonyl derivative, usually an aldehyde, ketone, or carboxylic acid, under acidic conditions to give an amine or amide, with expulsion of nitrogen. It is named after Karl Friedrich Schmidt (1887–1971), who first reported it in 1924 by successfully converting benzophenone and hydrazoic acid to benzanilide. The intramolecular reaction was not reported until 1991 but has become important in the synthesis of natural products. The reaction is effective with carboxylic acids to give amines (above), and with ketones to give amides (below). Reaction mechanism The reaction is closely related to the Curtius rearrangement except that in this reaction the acyl azide is produced by reaction of the carboxylic acid with hydrazoic acid via the protonated carboxylic acid, in a process akin to a Fischer esterification. An alternative, involving the formation of an acylium ion, becomes more im ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazoic Acid
Hydrazoic acid, also known as hydrogen azide, azic acid or azoimide, This also contains a detailed description of the contemporaneous production process. is a compound with the chemical formula . It is a colorless, volatile, and explosive liquid at room temperature and pressure. It is a compound of nitrogen and hydrogen, and is therefore a pnictogen hydride. It was first isolated in 1890 by Theodor Curtius. The acid has few applications, but its conjugate base, the azide ion, is useful in specialized processes. Hydrazoic acid, like its fellow mineral acids, is soluble in water. Undiluted hydrazoic acid is dangerously explosive with a standard enthalpy of formation ΔfHo (l, 298K) = +264 kJ/mol. When dilute, the gas and aqueous solutions (<10%) can be safely prepared but should be used immediately; because of its low boiling point, hydrazoic acid is enriched upon evaporation and condensation such that dilute solutions incapable of explosion can form droplets in the headspace of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
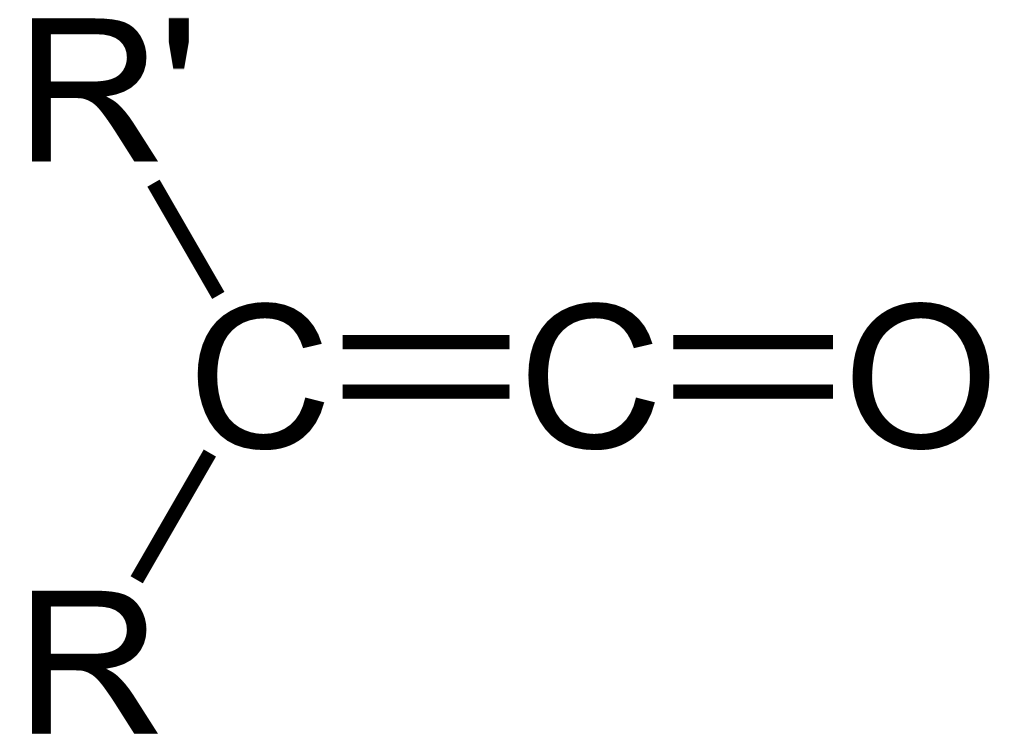
Ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary valence (chemistry), monovalent functional group, chemical groups (or two separate Substituent, substitution sites in the same molecule). The name may also refer to the specific compound ethenone , the simplest ketene. Although they are highly useful, most ketenes are chemical stability, unstable. When used as reagents in a chemical procedure, they are typically generated when needed, and consumed as soon as (or while) they are produced. History Ketenes were first studied as a class by Hermann Staudinger before 1905. Ketenes were systematically investigated by Hermann Staudinger in 1905 in the form of diphenylketene (conversion of \alpha-chlorodiphenyl acetyl chloride with zinc). Staudinger was inspired by the first examples of reactive organic intermediates and stable radicals discovered by Moses Gomberg in 1900 (compounds with triphenylmethyl group). Properties Ketenes are h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrosylsulfuric Acid
Nitrosylsulfuric acid is the chemical compound with the formula . It is a colourless solid that is used industrially in the production of caprolactam, and was formerly part of the lead chamber process for producing sulfuric acid. The compound is the mixed anhydride of sulfuric acid and nitrous acid. In organic chemistry, it is used as a reagent for nitrosating, as a diazotizing agent, and as an oxidizing agent. Synthesis and reactions A typical procedure entails dissolving sodium nitrite in cold sulfuric acid: : It can also be prepared by the reaction of nitric acid and sulfur dioxide. This procedure generates the nitrosylsulfuric acid as an intermediate en route to NOCl. is used in organic chemistry to prepare diazonium salts from amines, for example in the Sandmeyer reaction. Related NO-delivery reagents include nitrosonium tetrafluoroborate and nitrosyl chloride. In industry, the nitrosodecarboxylation reaction between nitrosylsulfuric acid and cyclohexanecarboxylic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexanecarboxylic Acid
Cyclohexanecarboxylic acid is the organic compound with the formula C6H11CO2H. It is the carboxylic acid of cyclohexane. It is a colorless oil that crystallizes near room temperature.. Preparation and reactions It is prepared by hydrogenation of benzoic acid. Cyclohexanecarboxylic acid is a precursor to the nylon-6 precursor caprolactam via its reaction with nitrosylsulfuric acid. It can also be oxidized to cyclohexene. Cyclohexanecarboxylic acid exhibits the reactions typical of carboxylic acids, including its conversion to the acid chloride cyclohexanecarbonyl chloride. Related compounds Derivatives related to cyclohexanecarboxylic acid include: * abscisic acid * buciclic acid * chlorogenic acid * chorismic acid * dicyclomine * quinic acid * shikimic acid * tranexamic acid Tranexamic acid is a medication used to treat or prevent excessive blood loss from major trauma, postpartum bleeding, surgery, tooth removal, nosebleeds, and heavy menstruation. It is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |