|
Radical Cation
Radical cations are denoted M^. Salts of these species have been isolated in the cases of dibenzocyclooctatetraene, various tertiary amines, and some polymethylated derivatives of azulene. Radical cations, like radical anions, have one unpaired electron, i.e. they are paramagnetic. Mass spectrometry Radical cations appear prominently in mass spectrometry. When a gas-phase molecule is subjected to electron ionization one electron is abstracted by an electron in the electron beam to create a radical cation M+.. This species represents the molecular ion or parent ion. A typical mass spectrum shows multiple signals because the molecular ion fragments into a complex mixture of ions and uncharged radical species. For example, the methanol radical cation fragments into a methenium cation and a hydroxyl radical. In naphthalene the unfragmented radical cation is by far the most prominent peak in the mass spectrum. Secondary species are generated from proton gain (M+1) and proton loss ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wurster's Cation
Tetramethylphenylenediamine (TMPD) is an organic compound with the formula . It is most studied of three isomers of this formula. It is a colorless solid. With two dimethylamino substituents, the ring is particularly electron rich. Redox behavior One-electron oxidation of TMPD gives the deep blue radical cation called Wurster's blue, . It was one of the first radical cations to be reported. Many properties have been described including its rapid rate of self-exchange: : X-ray crystallography of the TMPD and its iodide salt reveals that oxidation most strongly contracts the C-N(CH3)2 and the HC---CH bonds, indicating that oxidation gives a quinoid-like species. The monocation illustrates an early step in the oxidation of p-phenylenediamine derivatives, which are widely used for hair dying. N,N-Dimethylphenylenediamine () also easily oxidizes to a radical cation, called Wurster's Red. The hydrochloride salt of TMPD has been used as a redox indicator in the oxidase t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocycle
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of organic heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of organic heterocyclic chemistry focuses especially on organic unsaturated derivatives, and the preponderance of work and applications involves unstrained organic 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of organic heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanium
In chemistry, methanium is a complex positive ion with formula (metastable transitional form, a carbon atom covalently bonded to five hydrogen atoms) or (fluxional form, namely a molecule with one carbon atom covalently bonded to three hydrogen atoms and one dihydrogen molecule), bearing a +1 electric charge. It is a superacid and one of the onium ions, indeed the simplest carbonium ion. It is highly unstable and highly reactive even upon having a complete octet, thus granting its superacidic properties. Methanium can be produced in the laboratory as a rarefied gas or as a dilute species in superacids. It was prepared for the first time in 1950 and published in 1952 by Victor Talrose and his assistant Anna Konstantinovna Lyubimova. It occurs as an intermediate species in chemical reactions. The methanium ion is named after methane (), by analogy with the derivation of ammonium ion () from ammonia (). Structure Fluxional methanium can be visualised as a carbenium ion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonium Ion
In chemistry, a carbonium ion is a cation that has a pentacoordinated carbon atom. They are a type of carbocation. In older literature, the name "carbonium ion" was used for what is today called carbenium. Carbonium ions charge is delocalized in three-center, two-electron bonds. The more stable members are often bi- or polycyclic. 2-Norbornyl cation The 2-norbornyl cation is one of the best-characterized carbonium ions. It is the prototype for non-classical ions. As indicated first by low-temperature NMR spectroscopy and confirmed by X-ray crystallography, it has a symmetric structure with an RCH2+ group bonded to an alkene group, stabilized by a bicyclic structure. Cyclopropylmethyl cation A non-classical structure for is supported by substantial experimental evidence from solvolysis experiments and NMR studies. One or both of two structures, the cyclopropylcarbinyl cation and the bicyclobutonium cation, were invoked to account for the observed reactivity. The NMR spectr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbenium Ion
The carbenium ion is a kind of cation, positive ion with the structure RR′R″C+, that is, a chemical species with carbon atom having three covalent bonds, and it bears a +1 formal charge. Carbenium ions are a major subset of carbocations, which is a general term for diamagnetic carbon-based cations. In parallel with carbenium ions is another subset of carbocations, the carbonium ions with the formula R5+. In carbenium ions charge is localized. They are isoelectronic with monoboranes such as B(CH3)3. Nomenclature Reactivity Carbenium ions are generally highly reactive due to having an incomplete octet rule, octet of electrons; however, certain carbenium ions, such as the tropylium cation, tropylium ion, are relatively stable due to the positive charge being delocalised between the carbon atoms.(It can even exist stably in aqueous solution.) Rearrangements Carbenium ions sometimes rearrangement reaction, rearrange readily. For example, when pentan-3-ol is heated with aq ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central ion/molecule/atom is called a ligand. This number is determined somewhat differently for molecules than for crystals. For molecules and polyatomic ions the coordination number of an atom is determined by simply counting the other atoms to which it is bonded (by either single or multiple bonds). For example, [Cr(NH3)2Cl2Br2]− has Cr3+ as its central cation, which has a coordination number of 6 and is described as ''hexacoordinate''. The common coordination numbers are 4, 6 and 8. Molecules, polyatomic ions and coordination complexes In chemistry, coordination number, defined originally in 1893 by Alfred Werner, is the total number of neighbors of a central atom in a molecule or ion. The concept is most commonly applied to coordination ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other by the number of protons that are in their atoms. For example, any atom that contains 11 protons is sodium, and any atom that contains 29 protons is copper. Atoms with the same number of protons but a different number of neutrons are called isotopes of the same element. Atoms are extremely small, typically around 100 picometers across. A human hair is about a million carbon atoms wide. Atoms are smaller than the shortest wavelength of visible light, which means humans cannot see atoms with conventional microscopes. They are so small that accurately predicting their behavior using classical physics is not possible due to quantum mechanics, quantum effects. More than 99.94% of an atom's mass is in the nucleus. Protons hav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three Isotopes of carbon, isotopes occur naturally, carbon-12, C and carbon-13, C being stable, while carbon-14, C is a radionuclide, decaying with a half-life of 5,700 years. Carbon is one of the timeline of chemical element discoveries#Pre-modern and early modern discoveries, few elements known since antiquity. Carbon is the 15th abundance of elements in Earth's crust, most abundant element in the Earth's crust, and the abundance of the chemical elements, fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual abi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
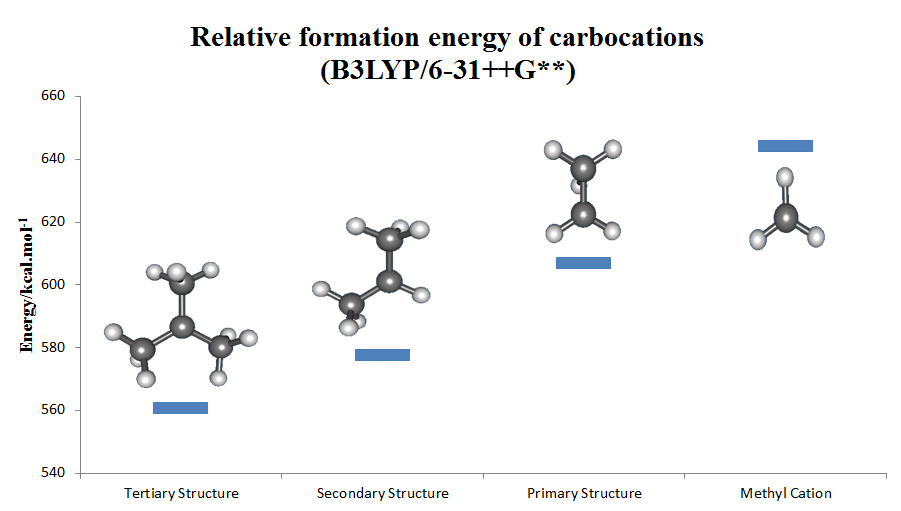
Carbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further classified in two main categories according to the coordination number of the charged carbon: three in the carbenium ions and five in the carbonium ions. Among the simplest carbocations are the methenium (a carbenium ion), methanium (a carbonium ion), acylium ions , and Vinyl cation, vinyl cations. Until the early 1970s, carbocations were called ''carbonium ions''. This nomenclature was proposed by George Andrew Olah, G. A. Olah. Carbonium ions, as originally defined by Olah, are characterized by a Three-center two-electron bond, three-center two-electron delocalized bonding scheme and are essentially synonymous with so-called 'non-classical carbocations', which are carbocations that contain bridging C–C or C–H σ-bonds. However ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bipolaron (chemistry)
In organic chemistry, bipolaron is a molecule or part of a macromolecular chain containing two positive charges in a conjugated system. The charges can be located in the centre of the chain or at its termini. Bipolarons and polarons are encountered in doped conducting polymers such as polythiophene. It is possible to synthesize and isolate bipolaron model compounds for X-ray diffraction studies. The diamagnetic bis(triaryl)amine dication 2 in ''scheme 1'' is prepared from the neutral precursor 1 in dichloromethane by reaction with 4 equivalents of antimony pentachloride. Two resonance structures exist for the dication. Structure 2a is a (singlet) diradical and 2b is the closed shell quinoid. The experimental bond lengths for the central vinylidene group in 2 are 141 pm and 137 pm compared to 144 pm and 134 pm for the precursor 1 implying some contribution from the quinoid structure. On the other hand, when a thiophene Thiophene is a heterocyclic compound with the formula C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polaron
A polaron is a quasiparticle used in condensed matter physics to understand the interactions between electrons and atoms in a solid material. The polaron concept was proposed by Lev Landau in 1933 and Solomon Pekar in 1946 to describe an electron moving in a dielectric crystal where the ions, atoms displace from their equilibrium positions to effectively screen the charge of an electron, known as a phonon cloud. This lowers the electron mobility and increases the electron's effective mass (solid-state physics), effective mass. The general concept of a polaron has been extended to describe other interactions between the electrons and ions in metals that result in a bound state, or a lowering of energy compared to the non-interacting system. Major theoretical work has focused on solving Herbert Fröhlich, Fröhlich and Holstein hamiltonian (quantum mechanics), Hamiltonians. This is still an active field of research to find exact numerical solutions to the case of one or two electro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferric Chloride
Iron(III) chloride describes the inorganic compounds with the formula (H2O)x. Also called ferric chloride, these compounds are some of the most important and commonplace compounds of iron. They are available both in anhydrous and in hydrated forms, which are both hygroscopic. They feature iron in its +3 oxidation state. The anhydrous derivative is a Lewis acid, while all forms are mild oxidizing agents. It is used as a water treatment, water cleaner and as an Chemical milling, etchant for metals. Electronic and optical properties All forms of ferric chloride are paramagnetic, owing to the presence of unpaired electrons residing in 3d orbitals. Although Fe(III) chloride can be octahedral or tetrahedral (or both, see structure section), all of these forms have five unpaired electrons, one per d-orbital. The spin states (d electrons), high spin d5 electronic configuration requires that d-d electronic transitions are spin forbidden, in addition to violating the Laporte rule. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |