|
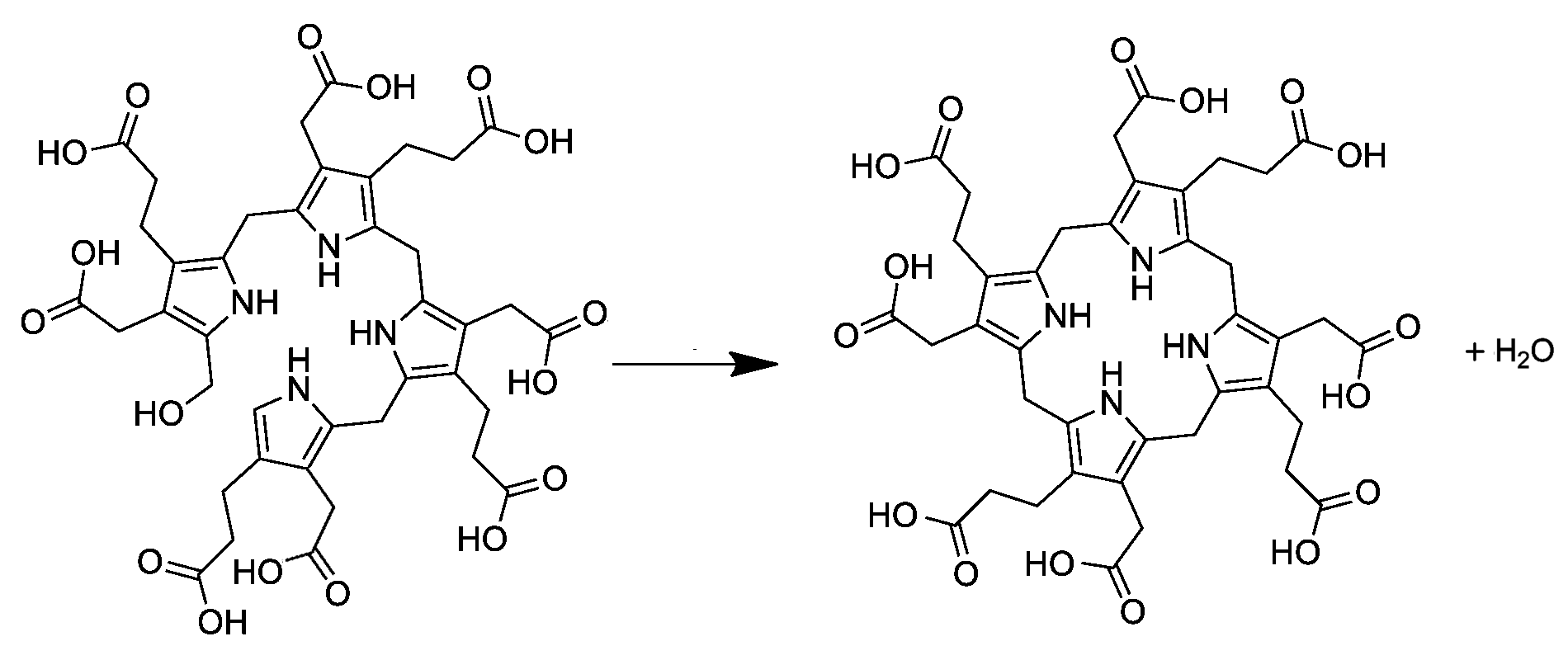
Preuroporphyrinogen
Hydroxymethylbilane, also known as preuroporphyrinogen, is an organic compound that occurs in living organisms during the synthesis of porphyrins, a group of critical substances that include haemoglobin, myoglobin, and chlorophyll. The name is often abbreviated as HMB. The compound is a substituted bilane, a chain of four pyrrole rings interconnected by methylene bridges . The chain starts with a hydroxymethyl group and ends with an hydrogen, in place of the respective methylene bridges. The other two carbon atoms of each pyrrole cycle are connected to an acetic acid group and a propionic acid group , in that order. The compound is generated from four molecules of porphobilinogen by the enzyme porphobilinogen deaminase: The enzyme uroporphyrinogen III synthase closes the chain to form a porphyrinogen a class of compounds with the hexahydroporphine macrocycle; specifically, uroporphyrinogen III. In the absence of the enzyme, the compound undergoes spontaneous cyclizat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uroporphyrinogen I
Uroporphyrinogen I is an isomer of uroporphyrinogen III, a metabolic intermediate in the biosynthesis of heme. A type of porphyria is caused by production of uroporphyrinogen I instead of III. Biosynthesis and metabolism In living organisms, uroporphyrinogen I occurs as a side branch of the main porphyrin synthesis pathway. In the normal pathway, the linear tetrapyrrole precursor preuroporphyrinogen (a substituted hydroxymethylbilane) is converted by the enzyme uroporphyrinogen-III cosynthase into the cyclic uroporphyrinogen III; which is then converted to coproporphyrinogen III on the way to porphyrins like heme. Uroporphyrinogen I is instead produced spontaneously from preuroporphyrinogen when the enzyme is not present. The difference between the I and III forms is the arrangement of the four carboxyethyl groups (propionic acid, "P") and the four carboxymethyl groups (acetic acid, "A"). The non-enzymatic conversion to uroporphyrinogen I results in the sequence AP-AP-AP-AP ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uroporphyrinogen III
Uroporphyrinogen III is a tetrapyrrole, the first macrocyclic intermediate in the biosynthesis of heme, chlorophyll, vitamin B12, and siroheme. It is a colorless compound, like other porphyrinogens. Structure The molecular structure of uroporphyrinogen III can be described as a hexahydroporphine core, where each pyrrole ring has the hydrogen atoms on its two outermost carbons replaced by an acetic acid group (, "A") and a propionic acid group (, "P"). The groups are attached in an asymmetric way: going around the macrocycle, the order is AP-AP-AP-PA. Biosynthesis and metabolism In the general porphyrin biosynthesis pathway, uroporphyrinogen III is derived from the linear tetrapyrrole preuroporphyrinogen (a substituted hydroxymethylbilane) by the action of the enzyme uroporphyrinogen-III cosynthase. The conversion entails a reversal of the last pyrrole unit (thus swapping the acetic and propionic acid groups) and a condensation reaction that closes the macrocycle by eliminati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bilane
In organic chemistry, bilane is a compound with the formula or . It is a tetrapyrrole, a class of compounds with four independent pyrrole rings. Specifically, the molecule can be described as four pyrrole molecules connected in an open chain by three methylene bridges at carbons adjacent to the nitrogens, replacing the respective hydrogens. The name is also used for the class of compounds formally derived from bilane proper by replacement of some additional hydrogen atoms by various functional groups. Natural bilanes usually have side chains substituted on the two carbons in each pyrrole ring that are not adjacent to the nitrogens. Artificial bilanes may be substituted on the bridging carbons (called ''meso'' positions). The parent (unsubstituted) bilane is difficult to prepare and unstable,Claudia Ryppa, Mathias O. Senge, Sabine S. Hatscher, Erich Kleinpeter, Philipp Wacker, Uwe Schilde, and Arno Wiehe (2005): "Synthesis of Mono‐ and Disubstituted Porphyrins: A‐ and 5 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
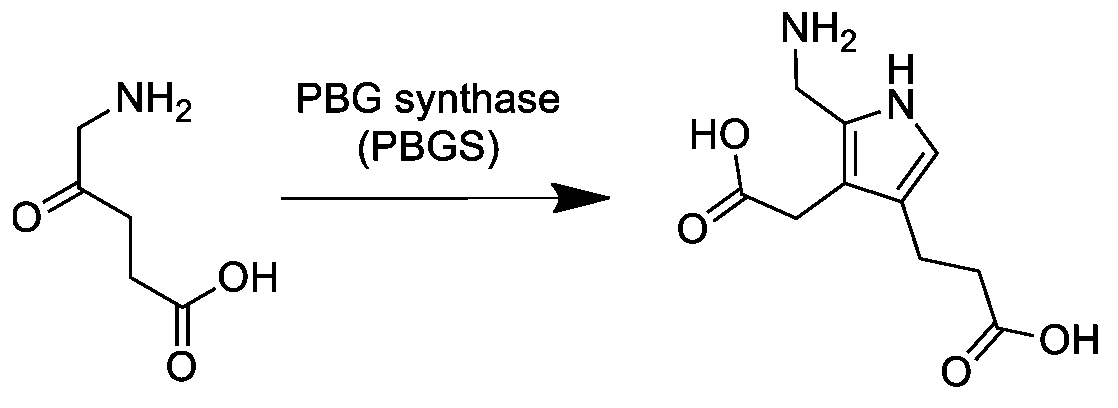
Porphobilinogen
Porphobilinogen (PBG) is an organic compound that occurs in living organisms as an intermediate in the biosynthesis of porphyrins, which include critical substances like hemoglobin and chlorophyll. The structure of the molecule can be described as molecule of pyrrole with sidechains substituted for hydrogen atoms at positions 2, 3 and 4 in the ring (1 being the nitrogen atom); respectively, an aminomethyl group , an acetic acid (carboxymethyl) group , and a propionic acid (carboxyethyl) group . Biosynthesis In the first step of the porphyrin biosynthesis pathway, porphobilinogen is generated from aminolevulinate (ALA) by the enzyme ALA dehydratase. Metabolism In the typical porphyrin biosynthesis pathway, four molecules of porphobilinogen are concatenated by carbons 2 and 5 of the pyrrole ring (adjacent to the nitrogen atom) into hydroxymethyl bilane by the enzyme porphobilinogen deaminase, also known as hydroxymethylbilane synthase. Pathologies Acute intermittent porphyr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexahydroporphine
Hexahydroporphine is an organic compound, organic chemical compound with formula . The molecule consists of four pyrrole rings connected by methylene bridges into a larger (non-aromatic (chemistry), aromatic) macrocycle ring, which makes it one of the simplest tetrapyrroles, and the simplest "true" one. As indicated by the name, it may be viewed as derived from porphine by the addition of six hydrogen atoms: four on the methine bridges, and two on the nitrogen atoms. Hexahydroporphine does not occur in nature, but is the core of porphyrinogens such as uroporphyrinogen III (UROGEN), which are precursors of many porphyrins — derivatives of porphine of great biological importance. The six hydrogens of that core are removed at a later metabolism, metabolic stage by the enzyme protoporphyrinogen oxidase. Because of this connection, the compound is also called (unsubstituted) porphyrinogen. The compound is a colorless solid, soluble in dichloromethane, acetone, and diethyl ether. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Porphyrinogen
In biochemistry a porphyrinogen is a member of a class of naturally occurring compounds with a tetrapyrrole core, a macrocycle Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry. ... of four pyrrole rings connected by four methylene bridges. - IUPAC Gold Book They can be viewed as derived from the parent compound hexahydroporphine by the substitution of various functional groups for hydrogen atoms in the outermost (20-carbon) ring. Porphyrinogens are intermediates in the biosynthesis of porphyrins, Cofactor (biochemistry), cofactors with a porphine core which are found in many enzymes and proteins including myoglobin, hemoglobin, cytochromes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uroporphyrinogen III Synthase
Uroporphyrinogen III synthase () is an enzyme involved in the metabolism of the cyclic tetrapyrrole compound porphyrin. It is involved in the conversion of hydroxymethyl bilane into uroporphyrinogen III. This enzyme catalyses the inversion of the final pyrrole unit (ring D) of the linear tetrapyrrole molecule, linking it to the first pyrrole unit (ring A), thereby generating a large macrocyclic structure, uroporphyrinogen III. The enzyme folds into two alpha/beta domains connected by a beta-ladder, the active site being located between the two domains. Pathology A deficiency is associated with Gunther's disease, also known as congenital erythropoietic porphyria (CEP). This is an autosomal recessive In genetics, dominance is the phenomenon of one variant (allele) of a gene on a chromosome masking or overriding the effect of a different variant of the same gene on the other copy of the chromosome. The first variant is termed dominant and t ... inborn error of metaboli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Porphobilinogen Deaminase
Porphobilinogen deaminase (hydroxymethylbilane synthase, or uroporphyrinogen I synthase) is an enzyme () that in humans is encoded by the HMBS gene. Porphobilinogen deaminase is involved in the third step of the heme biosynthetic pathway. It catalyzes the head to tail condensation of four porphobilinogen molecules into the linear hydroxymethylbilane while releasing four ammonia molecules: :4 porphobilinogen + H2O \rightleftharpoons hydroxymethylbilane + 4 NH3 Structure and function Functionally, porphobilinogen deaminase catalyzes the loss of ammonia from the porphobilinogen monomer (deamination) and its subsequent polymerization to a linear tetrapyrrole, which is released as hydroxymethylbilane: The structure of 40-42 kDa porphobilinogen deaminase, which is highly conserved amongst organisms, consists of three domains. Domains 1 and 2 are structurally very similar: each consisting of five beta-sheets and three alpha helices in humans. Domain 3 is positioned between the other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propionic Acid
Propionic acid (, from the Greek words πρῶτος : ''prōtos'', meaning "first", and πίων : ''píōn'', meaning "fat"; also known as propanoic acid) is a naturally occurring carboxylic acid with chemical formula CH3CH2CO2H. It is a liquid with a pungent and unpleasant smell somewhat resembling body odor. The anion CH3CH2CO2− as well as the salts and esters of propionic acid are known as propionates or propanoates. History Propionic acid was first described in 1844 by Johann Gottlieb, who found it among the degradation products of sugar. Over the next few years, other chemists produced propionic acid by different means, none of them realizing they were producing the same substance. In 1847, French chemist Jean-Baptiste Dumas established all the acids to be the same compound, which he called propionic acid, from the Greek words πρῶτος (prōtos), meaning ''first'', and πίων (piōn), meaning ''fat'', because it is the smallest H(CH2)''n''COOH acid that exhib ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |