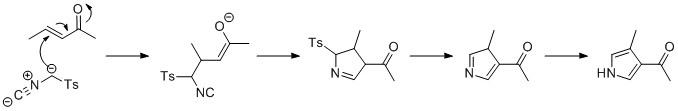
|
Polypyrrole
Polypyrrole (PPy) is an organic polymer obtained by oxidative polymerization of pyrrole. It is a solid with the formula H(C4H2NH)nH. It is an intrinsically conducting polymer, used in electronics, optical, biological and medical fields. History Some of the first examples of PPy were reported in 1919 by Angeli and Pieroni, who reported the formation of pyrrole blacks from pyrrole magnesium bromide. Since then pyrrole oxidation reaction has been studied and reported in scientific literature. Work on conductive polymers including polypyrrole, polythiophene, polyaniline, and polyacetylene was awarded the Nobel Prize in Chemistry in 2000 to Alan J. Heeger, Alan G. MacDiarmid and Hideki Shirakawa . Synthesis Different methods can be used to synthesize PPy, but the most common are electrochemical synthesis and chemical oxidation. Chemical oxidation of pyrrole: :n C4H4NH + 2n FeCl3 → (C4H2NH)n + 2n FeCl2 + 2n HCl The process is thought to occur via the formation of the p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4 H4 NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme. Pyrroles are components of more complex macrocycles, including the porphyrinogens and products derived therefrom, including porphyrins of heme, the chlorins, bacteriochlorins, and chlorophylls. Properties Pyrrole is a colorless volatile liquid that darkens readily upon exposure to air, and is usually purified by distillation immediately before use. Pyrrole has a nutty odor. Pyrrole is a 5-membered aromatic heterocycle, like furan and thiophene. Unlike furan and thiophene, it has a dipole in which the positive end lies on the side of the heteroatom, with a dipole moment of 1.58 D. In CDCl3, it ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrroles
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4 H4 NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme. Pyrroles are components of more complex macrocycles, including the porphyrinogens and products derived therefrom, including porphyrins of heme, the chlorins, bacteriochlorins, and chlorophylls. Properties Pyrrole is a colorless volatile liquid that darkens readily upon exposure to air, and is usually purified by distillation immediately before use. Pyrrole has a nutty odor. Pyrrole is a 5-membered aromatic heterocycle, like furan and thiophene. Unlike furan and thiophene, it has a dipole in which the positive end lies on the side of the heteroatom, with a dipole moment of 1.58 D. In CDCl3, it has ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrrole Electro-polymerisation
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4 H4 NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme. Pyrroles are components of more complex macrocycles, including the porphyrinogens and products derived therefrom, including porphyrins of heme, the chlorins, bacteriochlorins, and chlorophylls. Properties Pyrrole is a colorless volatile liquid that darkens readily upon exposure to air, and is usually purified by distillation immediately before use. Pyrrole has a nutty odor. Pyrrole is a 5-membered aromatic heterocycle, like furan and thiophene. Unlike furan and thiophene, it has a dipole in which the positive end lies on the side of the heteroatom, with a dipole moment of 1.58 D. In CDCl3, it has ch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conducting Polymer
Conductive polymers or, more precisely, intrinsically conducting polymers (ICPs) are organic polymers that conduct electricity. Such compounds may have metallic conductivity or can be semiconductors. The biggest advantage of conductive polymers is their processability, mainly by dispersion. Conductive polymers are generally not thermoplastics, ''i.e.'', they are not thermoformable. But, like insulating polymers, they are organic materials. They can offer high electrical conductivity but do not show similar mechanical properties to other commercially available polymers. The electrical properties can be fine-tuned using the methods of organic synthesis and by advanced dispersion techniques. History Polyaniline was first described in the mid-19th century by Henry Letheby, who investigated the electrochemical and chemical oxidation products of aniline in acidic media. He noted that reduced form was colourless but the oxidized forms were deep blue. The first highly-conductive organ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radical Cation
In organic chemistry, a radical anion is a free radical species that carries a negative charge. Radical anions are encountered in organic chemistry as reduced derivatives of polycyclic aromatic compounds, e.g. sodium naphthenide. An example of a non-carbon radical anion is the superoxide anion, formed by transfer of one electron to an oxygen molecule. Radical anions are typically indicated by M^. Polycyclic radical anions Many aromatic compounds can undergo one-electron reduction by alkali metals. The electron is transferred from the alkali metal ion to an unoccupied antibonding p-p п* orbital of the aromatic molecule. This transfer is usually only energetically favorable if the aprotic solvent efficiently solvates the alkali metal ion. Effective solvents are those that bind to the alkali metal cation: diethyl ether < < [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug Delivery
Drug delivery refers to approaches, formulations, manufacturing techniques, storage systems, and technologies involved in transporting a pharmaceutical compound to its target site to achieve a desired therapeutic effect. Principles related to drug preparation, route of administration, site-specific targeting, metabolism, and toxicity are used to optimize efficacy and safety, and to improve patient convenience and compliance. Drug delivery is aimed at altering a drug's pharmacokinetics and specificity by formulating it with different excipients, drug carriers, and medical devices. There is additional emphasis on increasing the bioavailability and duration of action of a drug to improve therapeutic outcomes. Some research has also been focused on improving safety for the person administering the medication. For example, several types of microneedle patches have been developed for administering vaccines and other medications to reduce the risk of needlestick injury. Drug deli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Semiconductors
Organic semiconductors are solids whose building blocks are pi-bonded molecules or polymers made up by carbon and hydrogen atoms and – at times – heteroatoms such as nitrogen, sulfur and oxygen. They exist in the form of molecular crystals or amorphous thin films. In general, they are electrical insulators, but become semiconducting when charges are either injected from appropriate electrodes, upon doping or by photoexcitation. General properties In molecular crystals the energetic separation between the top of the valence band and the bottom conduction band, i.e. the band gap, is typically 2.5–4 eV, while in inorganic semiconductors the band gaps are typically 1–2 eV. This implies that they are, in fact, insulators rather than semiconductors in the conventional sense. They become semiconducting only when charge carriers are either injected from the electrodes or generated by intentional or unintentional doping. Charge carriers can also be generated in the cou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Electronics
Molecular electronics is the study and application of molecular building blocks for the fabrication of electronic components. It is an interdisciplinary area that spans physics, chemistry, and materials science. The unifying feature is use of molecular building blocks to fabricate electronic components. Due to the prospect of size reduction in electronics offered by molecular-level control of properties, molecular electronics has generated much excitement. It provides a potential means to extend Moore's Law beyond the foreseen limits of small-scale conventional silicon integrated circuits. Molecular scale electronics Molecular scale electronics, also called single-molecule electronics, is a branch of nanotechnology that uses single molecules, or nanoscale collections of single molecules, as electronic components. Because single molecules constitute the smallest stable structures possible, this miniaturization is the ultimate goal for shrinking electrical circuits. Conventiona ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Polymers
A polymer (; Greek '' poly-'', "many" + '' -mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals. The term "polymer" derives from the Greek word πολύς (''polus'', meaning "many, much") and μέρος (''meros'', meaning "p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrapyrrole
Tetrapyrroles are a class of chemical compounds that contain four pyrrole or pyrrole-like rings. The pyrrole/pyrrole derivatives are linked by ( =- or -- units), in either a linear or a cyclic fashion. Pyrroles are a five-atom ring with four carbon atoms and one nitrogen atom. Tetrapyrroles are common cofactors in biochemistry and their biosynthesis and degradation feature prominently in the chemistry of life. Some tetrapyrroles form the active core of compounds with crucial biochemical roles in living systems, such as hemoglobin and chlorophyll. In these two molecules, in particular, the pyrrole macrocycle ring frames a metal atom, that forms a coordination compound with the pyrroles and plays a central role in the biochemical function of those molecules. Structure Linear tetrapyrroles (called bilanes) include: *Heme breakdown products (e.g., bilirubin, biliverdin) *Phycobilins (found in cyanobacteria) *Luciferins as found in dinoflagellates and euphausiid shrimps (krill) F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Semiconductor
Organic semiconductors are solids whose building blocks are pi-bonded molecules or polymers made up by carbon and hydrogen atoms and – at times – heteroatoms such as nitrogen, sulfur and oxygen. They exist in the form of molecular crystals or amorphous thin films. In general, they are electrical insulators, but become semiconducting when charges are either injected from appropriate electrodes, upon doping or by photoexcitation. General properties In molecular crystals the energetic separation between the top of the valence band and the bottom conduction band, i.e. the band gap, is typically 2.5–4 eV, while in inorganic semiconductors the band gaps are typically 1–2 eV. This implies that they are, in fact, insulators rather than semiconductors in the conventional sense. They become semiconducting only when charge carriers are either injected from the electrodes or generated by intentional or unintentional doping. Charge carriers can also be generated in the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |