|
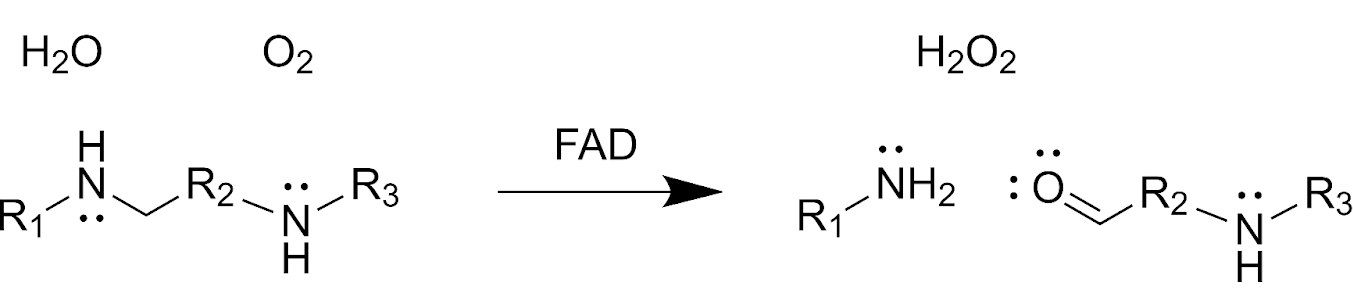
Polyamine Oxidase
A polyamine oxidase (PAO) is an enzymatic flavoprotein that oxidizes a carbon-nitrogen bond in a secondary amino group of a polyamine donor, using molecular oxygen as an acceptor. The generalized PAO reaction converts three substrates (water, oxygen, and a polyamine with both primary and secondary amino groups) into three products (hydrogen peroxide, an amino-aldehyde, and a primary amine). Different PAOs with varying substrate specificities exist in different organisms. Phylogenetic analyses suggest that PAOs likely evolved once in eukaryotes and diversified by divergent evolution and gene duplication events, though some prokaryotes have acquired PAOs through horizontal gene transfer. Structure and Mechanism Structures of PAOs from corn, brewer’s yeast, and mice contain a substrate-binding domain and an FAD-binding domain that secures the FAD cofactor non-covalently. The active site is located at the interface of these domains. Active sites in PAOs vary, but some feat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the react ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the deprotonated –COO− form under biological conditions), and an imidazole side chain (which is partially protonated), classifying it as a positively charged amino acid at physiological pH. Initially thought essential only for infants, it has now been shown in longer-term studies to be essential for adults also. It is encoded by the codons CAU and CAC. Histidine was first isolated by Albrecht Kossel and Sven Gustaf Hedin in 1896. It is also a precursor to histamine, a vital inflammatory agent in immune responses. The acyl radical is histidyl. Properties of the imidazole side chain The conjugate acid (protonated form) of the imidazole side chain in histidine has a p''K''a of approximately 6.0. Thus, below a pH of 6, the imidazole ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavin Enzymes
Flavin may refer to: Placename * Flavin, Aveyron, a commune in southern France Surname * Adrian Flavin (born 1979), a professional rugby player * Christopher Flavin, president of the Worldwatch Institute * Dan Flavin (1933–1996), a minimalist artist famous for using fluorescent light fixtures * Dan Flavin (politician), Louisiana politician * James Flavin (1906–1976), an American character actor * Jennifer Flavin (born 1968), a former model and wife of actor Sylvester Stallone * Martin Flavin (1883–1967), an American playwright and novelist * Martin Flavin (politician) (1841–1917), Irish Nationalist politician, Member of Parliament (MP) for Cork, 1891–1892 * Michael Joseph Flavin (1866-1944), Irish Nationalist politician, Member of Parliament (MP) for North Kerry, 1896-1918 * Mick Flavin, an Irish country singer Biochemistry * Flavin adenine dinucleotide (FAD), a redox cofactor * Flavin-containing amine oxidoreductase, a family of amine oxidases * Flavin-conta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MTOR Complex 1
mTORC1, also known as mammalian target of rapamycin complex 1 or mechanistic target of rapamycin complex 1, is a protein complex that functions as a nutrient/energy/redox sensor and controls protein synthesis. mTOR Complex 1 (mTORC1) is composed of the mTOR protein complex, regulatory-associated protein of mTOR (commonly known as raptor), mammalian lethal with SEC13 protein 8 ( MLST8), PRAS40 and DEPTOR. This complex embodies the classic functions of mTOR, namely as a nutrient/energy/redox sensor and controller of protein synthesis. The activity of this complex is regulated by rapamycin, insulin, growth factors, phosphatidic acid, certain amino acids and their derivatives (e.g., -leucine and β-hydroxy β-methylbutyric acid), mechanical stimuli, and oxidative stress. Recently it has been also demonstrated that cellular bicarbonate metabolism can be regulated by mTORC1 signaling. The role of mTORC1 is to activate translation of proteins. In order for cells to grow and pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Initiation Factor
Initiation factors are proteins that bind to the small subunit of the ribosome during the initiation of translation, a part of protein biosynthesis. Initiation factors can interact with repressors to slow down or prevent translation. They have the ability to interact with activators to help them start or increase the rate of translation. In bacteria, they are simply called IFs (i.e.., IF1, IF2, & IF3) and in eukaryotes they are known as eIFs (i.e.., eIF1, eIF2, eIF3). Translation initiation is sometimes described as three step process by which initiation factors help to carry out. First, the tRNA carrying a methionine amino acid binds to the small ribosome, then binds to the mRNA, and finally joining together with the large ribosome. The initiation factors that help with this process each have different roles and structures. Types The initiation factors are divided into three major groups by taxonomic domains. There are some homologies shared (click the domain names to see ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypusine
Hypusine is an uncommon amino acid found in all eukaryotes and in some archaea, but not in bacteria. The only known proteins containing the hypusine residue is eukaryotic translation initiation factor 5A (eIF-5A) and a similar protein found in archaea. In humans, two isoforms of eIF-5A have been described: eIF5A-1 and eIF5A-2. They are encoded by two distinct genes EIF5A and EIF5A2. The protein is involved in protein biosynthesis and promotes the formation of the first peptide bond. The region surrounding the hypusine residue is highly conserved and is essential to the function of eIF5A. Thus, hypusine and eIF-5A appear to be vital for the viability and proliferation of eukaryotic cells. Hypusine is formed in eIF-5A by post-translational modification of one of the lysyl residues. Two reactions and two enzymes are involved: *1. Deoxyhypusine synthase catalyzes the cleavage of the polyamine spermidine and transfer of its 4-aminobutyl moiety to the ε-amino group of one specifi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Programmed Cell Death
Programmed cell death (PCD; sometimes referred to as cellular suicide) is the death of a cell (biology), cell as a result of events inside of a cell, such as apoptosis or autophagy. PCD is carried out in a biological process, which usually confers advantage during an organism's biological life cycle, lifecycle. For example, the Limb development, differentiation of fingers and toes in a developing human embryo occurs because cells between the fingers apoptose; the result is that the digits are separate. PCD serves fundamental functions during both plant and animal tissue development. Apoptosis and autophagy are both forms of programmed cell death. Necrosis is the death of a cell caused by external factors such as trauma or infection and occurs in several different forms. Necrosis was long seen as a non-physiological process that occurs as a result of infection or injury, but in the 2000s, a form of programmed necrosis, called necroptosis, was recognized as an alternative form of pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactive Oxygen Species
In chemistry, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen (). Examples of ROS include peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha-oxygen. The reduction of molecular oxygen () produces superoxide (), which is the precursor to most other reactive oxygen species: :O2 + e^- -> \ ^\bullet O2- Dismutation of superoxide produces hydrogen peroxide (): :2 H+ + \ ^\bullet O2^- + \ ^\bullet O2^- -> H2O2 + O2 Hydrogen peroxide in turn may be partially reduced, thus forming hydroxide ions and hydroxyl radicals (), or fully reduced to water: :H2O2 + e^- -> HO^- + \ ^\bullet OH :2 H+ + 2 e- + H2O2 -> 2 H2O In a biological context, ROS are byproducts of the normal metabolism of oxygen. ROS have roles in cell signaling and homeostasis. ROS are intrinsic to cellular functioning, and are present at low and stationary levels in normal cells. In plants, ROS are involved in metabolic processes related to photoprotection and toleran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catabolism
Catabolism () is the set of metabolic pathways that breaks down molecules into smaller units that are either oxidized to release energy or used in other anabolic reactions. Catabolism breaks down large molecules (such as polysaccharides, lipids, nucleic acids, and proteins) into smaller units (such as monosaccharides, fatty acids, nucleotides, and amino acids, respectively). Catabolism is the breaking-down aspect of metabolism, whereas anabolism is the building-up aspect. Cells use the monomers released from breaking down polymers to either construct new polymer molecules or degrade the monomers further to simple waste products, releasing energy. Cellular wastes include lactic acid, acetic acid, carbon dioxide, ammonia, and urea. The formation of these wastes is usually an oxidation process involving a release of chemical free energy, some of which is lost as heat, but the rest of which is used to drive the synthesis of adenosine triphosphate (ATP). This molecule ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
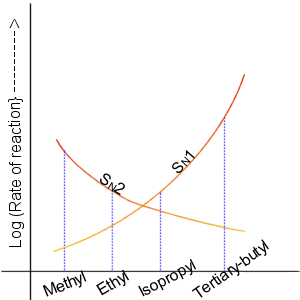
Nucleophilic Substitution
In chemistry, a nucleophilic substitution is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile). The molecule that contains the electrophile and the leaving functional group is called the substrate. The most general form of the reaction may be given as the following: :\text\mathbf + \ce + \text\mathbf The electron pair (:) from the nucleophile (Nuc) attacks the substrate () and bonds with it. Simultaneously, the leaving group (LG) departs with an electron pair. The principal product in this case is . The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. An example of nucleophilic substitution is the hydrolysis of an alkyl bromide, R-Br under basic conditions, where the attacking nucleophile is hydroxyl () and the leaving group is bromide (). :R-Br + OH ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |