|
Photo Etching
Photoengraving is a process that uses a light-sensitive photoresist applied to the surface to be engraved to create a mask that protects some areas during a subsequent operation which etches, dissolves, or otherwise removes some or all of the material from the unshielded areas of a substrate. Normally applied to metal, it can also be used on glass, plastic and other materials. A photoresist is selected which is resistant to the particular acid or other etching compound to be used. It may be a liquid applied by brushing, spraying, pouring or other means and then allowed to set, or it may come in sheet form and be applied by laminating. It is then exposed to light—usually strong ultraviolet (UV) light—through a photographic, mechanically printed, or manually created image or pattern on transparent film. Alternatively, a lens may be used to project an image directly onto it. Typically, the photoresist is hardened where it receives sufficient exposure to light, but some photoresis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoresist
A photoresist (also known simply as a resist) is a light-sensitive material used in several processes, such as photolithography and photoengraving, to form a patterned coating on a surface. This process is crucial in the electronic industry. The process begins by coating a substrate with a light-sensitive organic material. A patterned mask is then applied to the surface to block light, so that only unmasked regions of the material will be exposed to light. A solvent, called a developer, is then applied to the surface. In the case of a positive photoresist, the photo-sensitive material is degraded by light and the developer will dissolve away the regions that were exposed to light, leaving behind a coating where the mask was placed. In the case of a negative photoresist, the photosensitive material is strengthened (either polymerized or cross-linked) by light, and the developer will dissolve away only the regions that were not exposed to light, leaving behind a coating in areas w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typically ductile (can be drawn into wires) and malleable (they can be hammered into thin sheets). These properties are the result of the ''metallic bond'' between the atoms or molecules of the metal. A metal may be a chemical element such as iron; an alloy such as stainless steel; or a molecular compound such as polymeric sulfur nitride. In physics, a metal is generally regarded as any substance capable of conducting electricity at a temperature of absolute zero. Many elements and compounds that are not normally classified as metals become metallic under high pressures. For example, the nonmetal iodine gradually becomes a metal at a pressure of between 40 and 170 thousand times atmospheric pressure. Equally, some materials regarded as metals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
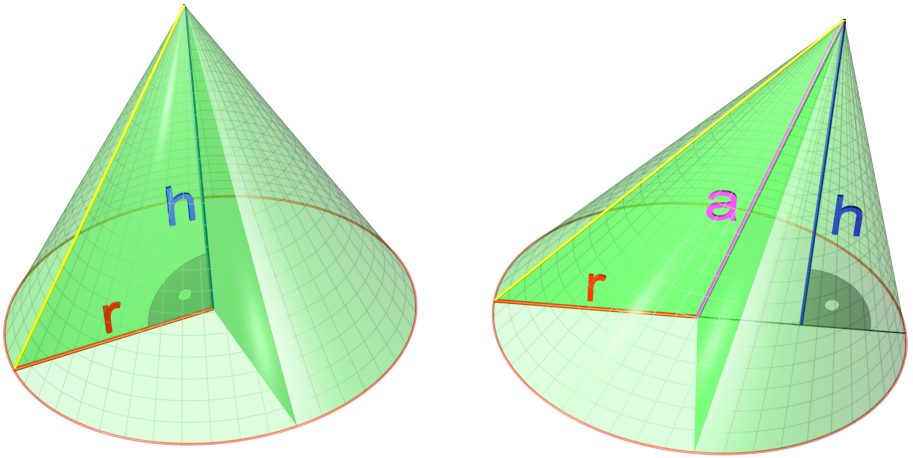
Cone (geometry)
A cone is a three-dimensional geometric shape that tapers smoothly from a flat base (frequently, though not necessarily, circular) to a point called the apex or vertex. A cone is formed by a set of line segments, half-lines, or lines connecting a common point, the apex, to all of the points on a base that is in a plane that does not contain the apex. Depending on the author, the base may be restricted to be a circle, any one-dimensional quadratic form in the plane, any closed one-dimensional figure, or any of the above plus all the enclosed points. If the enclosed points are included in the base, the cone is a solid object; otherwise it is a two-dimensional object in three-dimensional space. In the case of a solid object, the boundary formed by these lines or partial lines is called the ''lateral surface''; if the lateral surface is unbounded, it is a conical surface. In the case of line segments, the cone does not extend beyond the base, while in the case of half-lin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Soap
Soap is a salt of a fatty acid used in a variety of cleansing and lubricating products. In a domestic setting, soaps are surfactants usually used for washing, bathing, and other types of housekeeping. In industrial settings, soaps are used as thickeners, components of some lubricants, and precursors to catalysts. When used for cleaning, soap solubilizes particles and grime, which can then be separated from the article being cleaned. In hand washing, as a surfactant, when lathered with a little water, soap kills microorganisms by disorganizing their membrane lipid bilayer and denaturing their proteins. It also emulsifies oils, enabling them to be carried away by running water. Soap is created by mixing fats and oils with a base. A similar process is used for making detergent which is also created by combining chemical compounds in a mixer. Humans have used soap for millennia. Evidence exists for the production of soap-like materials in ancient Babylon around 2800 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% , it is referred to as ''fuming nitric acid''. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%. Nitric acid is the primary reagent used for nitration – the addition of a nitro group, typically to an organic molecule. While some resulting nitro compounds are shock- and thermally-sensitive explosives, a few are stable enough to be used in munitions and demolition, while others are still more stable and used as pigments in inks and dyes. Nitric acid is also commonly used as a strong oxidizing agen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic table) it occurs naturally only in combination with other elements and it almost always has an oxidation state of +2. It reacts readily with air to form a thin passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight alloys that contain aluminium. In the cosmos, magnesium is produced in large, aging stars by the sequential addition of three helium nuclei to a carbon nucleus. When such stars explode as supernovas, much of the magnesium is expelled into the interstellar medium where it ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic table. In some respects, zinc is chemically similar to magnesium: both elements exhibit only one normal oxidation state (+2), and the Zn2+ and Mg2+ ions are of similar size.The elements are from different metal groups. See periodic table. Zinc is the 24th most abundant element in Earth's crust and has five stable isotopes. The most common zinc ore is sphalerite (zinc blende), a zinc sulfide mineral. The largest workable lodes are in Australia, Asia, and the United States. Zinc is refined by froth flotation of the ore, roasting, and final extraction using electricity ( electrowinning). Zinc is an essential trace element for humans, animals, plants and for microorganisms and is necessary for prenatal and postnatal development. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spray-paint
Spray painting is a painting technique in which a device sprays coating material (paint, ink, varnish, etc.) through the air onto a surface. The most common types employ compressed gas—usually air—to atomize and direct the paint particles. Spray guns evolved from airbrushes, and the two are usually distinguished by their size and the size of the spray pattern they produce. Airbrushes are hand-held and used instead of a brush for detailed work such as photo retouching, painting nails, or fine art. Air gun spraying uses generally larger equipment. It is typically used for covering large surfaces with an even coating of liquid. Spray guns can be either automated or hand-held and have interchangeable heads to allow for different spray patterns. Single color aerosol paint cans are portable and easy to store. History Spraying paint with compressed air can be traced back to its use on the Southern Pacific Railway in the early 1880s In 1887 Joseph Binks, the maintenance supervisor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in sea water, aqua regia, and chlorine. Titanium was discovered in Cornwall, Great Britain, by William Gregor in 1791 and was named by Martin Heinrich Klaproth after the Titans of Greek mythology. The element occurs within a number of minerals, principally rutile and ilmenite, which are widely distributed in the Earth's crust and lithosphere; it is found in almost all living things, as well as bodies of water, rocks, and soils. The metal is extracted from its principal mineral ores by the Kroll and Hunter processes. The most common compound, titanium dioxide, is a popular photocatalyst and is used in the manufacture of white pigments. Other compounds include titanium tetrachloride (TiCl4), a component of smoke screens and catalysts; and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferric Chloride
Iron(III) chloride is the inorganic compound with the formula . Also called ferric chloride, it is a common compound of iron in the +3 oxidation state. The anhydrous compound is a crystalline solid with a melting point of 307.6 °C. The colour depends on the viewing angle: by reflected light the crystals appear dark green, but by transmitted light they appear purple-red. Structure and properties Anhydrous Anhydrous iron(III) chloride has the structure, with octahedral Fe(III) centres interconnected by two-coordinate chloride ligands. Iron(III) chloride has a relatively low melting point and boils at around 315 °C. The vapor consists of the dimer (like aluminium chloride) which increasingly dissociates into the monomeric (with D3h point group molecular symmetry) at higher temperature, in competition with its reversible decomposition to give iron(II) chloride and chlorine gas. Hydrates In addition to the anhydrous material, ferric chloride forms four hydrates. All f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(III) Chloride
Iron(III) chloride is the inorganic compound with the formula . Also called ferric chloride, it is a common compound of iron in the +3 oxidation state. The anhydrous compound is a crystalline solid with a melting point of 307.6 °C. The colour depends on the viewing angle: by reflected light the crystals appear dark green, but by transmitted light they appear purple-red. Structure and properties Anhydrous Anhydrous iron(III) chloride has the structure, with octahedral Fe(III) centres interconnected by two-coordinate chloride ligands. Iron(III) chloride has a relatively low melting point and boils at around 315 °C. The vapor consists of the dimer (like aluminium chloride) which increasingly dissociates into the monomeric (with D3h point group molecular symmetry) at higher temperature, in competition with its reversible decomposition to give iron(II) chloride and chlorine gas. Hydrates In addition to the anhydrous material, ferric chloride forms four hydrates. All ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brass
Brass is an alloy of copper (Cu) and zinc (Zn), in proportions which can be varied to achieve different mechanical, electrical, and chemical properties. It is a substitutional alloy: atoms of the two constituents may replace each other within the same crystal structure. Brass is similar to bronze, another copper alloy, that uses tin instead of zinc. Both bronze and brass may include small proportions of a range of other elements including arsenic (As), lead (Pb), phosphorus (P), aluminium (Al), manganese (Mn), and silicon (Si). Historically, the distinction between the two alloys has been less consistent and clear, and modern practice in museums and archaeology increasingly avoids both terms for historical objects in favor of the more general "copper alloy". Brass has long been a popular material for decoration due to its bright, gold-like appearance; being used for drawer pulls and doorknobs. It has also been widely used to make utensils because of its low melting ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |