|
Phenylpropanoids
The phenylpropanoids are a diverse family of organic compounds that are synthesized by plants from the amino acids phenylalanine and tyrosine. Their name is derived from the six-carbon, aromatic phenyl group and the three-carbon propene tail of coumaric acid, which is the central intermediate in phenylpropanoid biosynthesis. From 4-coumaroyl-CoA emanates the biosynthesis of myriad natural products including lignols (precursors to lignin and lignocellulose), flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids. The coumaroyl component is produced from cinnamic acid. Phenylpropanoids are found throughout the plant kingdom, where they serve as essential components of a number of structural polymers, provide protection from ultraviolet light, defend against herbivores and pathogens, and also mediate plant-pollinator interactions as floral pigments and scent compounds. Hydroxycinnamic acids Phenylalanine is first converted to cinnamic acid by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lignin
Lignin is a class of complex organic polymers that form key structural materials in the support tissues of most plants. Lignins are particularly important in the formation of cell walls, especially in wood and bark, because they lend rigidity and do not rot easily. Chemically, lignins are polymers made by cross-linking phenolic precursors. History Lignin was first mentioned in 1813 by the Swiss botanist A. P. de Candolle, who described it as a fibrous, tasteless material, insoluble in water and alcohol but soluble in weak alkaline solutions, and which can be precipitated from solution using acid. He named the substance “lignine”, which is derived from the Latin word ''lignum'', meaning wood. It is one of the most abundant organic polymers on Earth, exceeded only by cellulose. Lignin constitutes 30% of non-fossil organic carbon on Earth, and 20 to 35% of the dry mass of wood. Lignin is present in red algae, which suggest that the common ancestor of plants and red algae als ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavonoids
Flavonoids (or bioflavonoids; from the Latin word ''flavus'', meaning yellow, their color in nature) are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans. Chemically, flavonoids have the general structure of a 15-carbon skeleton, which consists of two phenyl rings (A and B) and a heterocyclic ring (C, the ring containing the embedded oxygen). This carbon structure can be abbreviated C6-C3-C6. According to the IUPAC nomenclature, they can be classified into: *flavonoids or bioflavonoids *isoflavonoids, derived from 3-phenyl chromen-4-one (3-phenyl-1,4-benzopyrone) structure * neoflavonoids, derived from 4-phenylcoumarine (4-phenyl-1,2-benzopyrone) structure The three flavonoid classes above are all ketone-containing compounds and as such, anthoxanthins ( flavones and flavonols). This class was the first to be termed bioflavonoids. The terms flavonoid and bioflavonoid have also been more loosely used to describe n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cinnamic Acid
Cinnamic acid is an organic compound with the formula C6H5-CH=CH- COOH. It is a white crystalline compound that is slightly soluble in water, and freely soluble in many organic solvents. Classified as an unsaturated carboxylic acid, it occurs naturally in a number of plants. It exists as both a ''cis'' and a ''trans'' isomer, although the latter is more common. Occurrence and production Biosynthesis Cinnamic acid is a central intermediate in the biosynthesis of a myriad of natural products including lignols (precursors to lignin and lignocellulose), flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids. Its biosynthesis involves the action of the enzyme phenylalanine ammonia-lyase (PAL) on phenylalanine. Natural occurrence It is obtained from oil of cinnamon, or from balsams such as storax. It is also found in shea butter. Cinnamic acid has a honey-like odor; it and its more volatile ethyl ester ( ethyl cinnamate) are flavor components in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stilbenes
Stilbenoids are hydroxylated derivatives of stilbene. They have a C6–C2–C6 structure. In biochemical terms, they belong to the family of phenylpropanoids and share most of their biosynthesis pathway with chalcones. Most stilbenoids are produced by plants, and the only known exception is the antihelminthic and antimicrobial stilbenoid, 2-isopropyl-5- ''E'')-2-phenylvinylenzene-1,3-diol, biosynthesized by the Gram-negative bacterium ''Photorhabdus luminescens.'' Chemistry Stilbenoids are hydroxylated derivatives of stilbene and have a C6–C2–C6 structure. They belong to the family of phenylpropanoids and share most of their biosynthesis pathway with chalcones. Under UV irradiation, stilbene and its derivatives undergo intramolecular cyclization, called stilbene photocyclization to form dihydrophenanthrenes. Oligomeric forms are known as oligostilbenoids. Types ;Aglycones * Piceatannol in the roots of Norway spruces * Pinosylvin is a fungal toxin protecting wood from fun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coumarin
Coumarin () or 2''H''-chromen-2-one is an aromatic organic chemical compound with formula . Its molecule can be described as a benzene molecule with two adjacent hydrogen atoms replaced by a lactone-like chain , forming a second six-membered heterocycle that shares two carbons with the benzene ring. It can be placed in the benzopyrone chemical class and considered as a lactone. Coumarin is a colorless crystalline solid with a sweet odor resembling the scent of vanilla and a bitter taste. It is found in many plants, where it may serve as a chemical defense against predators. By inhibiting synthesis of vitamin K, a related compound is used as the prescription drug warfarin – an anticoagulant – to inhibit formation of blood clots, deep vein thrombosis, and pulmonary embolism. Etymology Coumarin is derived from ''coumarou'', the French word for the tonka bean. The word ''tonka'' for the tonka bean is taken from the Galibi (Carib) tongue spoken by natives of French Gui ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
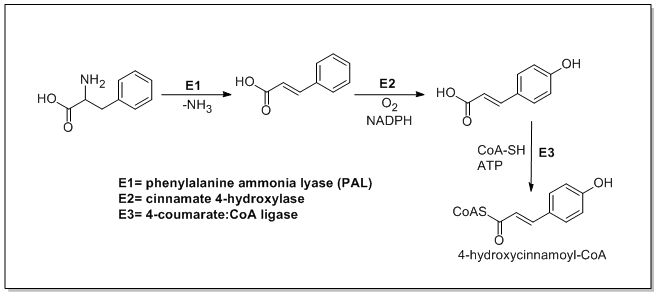
Phenylpropanoid Biosynthesis
The biosynthesis of phenylpropanoids involves a number of enzymes. From amino acids to cinnamates In plants, all phenylpropanoids are derived from the amino acids phenylalanine and tyrosine. Phenylalanine ammonia-lyase (PAL, a.k.a. phenylalanine/tyrosine ammonia-lyase) is an enzyme that transforms L-phenylalanine and tyrosine into trans-cinnamic acid and ''p''-coumaric acid, respectively. Trans-cinnamate 4-monooxygenase (cinnamate 4-hydroxylase) is the enzyme that transforms trans-cinnamate into 4-hydroxycinnamate (''p''-coumaric acid). 4-Coumarate-CoA ligase is the enzyme that transforms 4-coumarate (''p''-coumaric acid) into 4-coumaroyl-CoA. Enzymes associated with biosynthesis of hydroxycinnamic acids * Cinnamyl-alcohol dehydrogenase (CAD), an enzyme that transforms cinnamyl alcohol into cinnamaldehyde * Sinapine esterase, an enzyme that transforms sinapoylcholine into sinapate ( sinapic acid) and choline * Trans-cinnamate 2-monooxygenase, an enzyme that transforms tra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monolignol
Monolignols, also called lignols, are the source materials for biosynthesis of both lignans and lignin and consist mainly of paracoumaryl alcohol (H), coniferyl alcohol (G) and sinapyl alcohol (S). These monolignols differ in their degree of methoxilation of the aromatic ring. The monolignols are derived from the amino acid phenylalanine via the phenylpropanoid pathway involving various enzymes. Phenylalanine is first converted to paracoumaryl alcohol (H), which is subsequently elaborated to coniferyl alcohol (G) and sinapyl alcohol (S). This reaction happens in the cytosol, while the polymerization of the monolignols occurs in the apoplast to which the monolignols have to be transported to though the cell membrane. The monolignols have been found as monolignol-4-O-β-d- glucosides, which might be their major way of storage. Another theory for this conversion is that is improving the transportation of the monolignols. The polymerization consists of oxidative coupling reacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylalanine Ammonia-lyase
The enzyme phenylalanine ammonia lyase (EC 4.3.1.24) catalyzes the conversion of L-phenylalanine to ammonia and ''trans''-cinnamic acid.: :L-phenylalanine = ''trans''-cinnamate + NH3 Phenylalanine ammonia lyase (PAL) is the first and committed step in the phenyl propanoid pathway and is therefore involved in the biosynthesis of the polyphenol compounds such as flavonoids, phenylpropanoids, and lignin in plants. Phenylalanine ammonia lyase is found widely in plants, as well as some bacteria, yeast, and fungi, with isoenzymes existing within many different species. It has a molecular mass in the range of 270–330 kDa. The activity of PAL is induced dramatically in response to various stimuli such as tissue wounding, pathogenic attack, light, low temperatures, and hormones. PAL has recently been studied for possible therapeutic benefits in humans afflicted with phenylketonuria. It has also been used in the generation of L-phenylalanine as precursor of the sweetener aspar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the react ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-Coumaroyl-CoA
Coumaroyl-coenzyme A is the thioester of coenzyme-A and coumaric acid. Coumaroyl-coenzyme A is a central intermediate in the biosynthesis of myriad natural products found in plants. These products include lignols (precursors to lignin and lignocellulose), flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and other phenylpropanoids. Biosynthesis and significance It is generated in nature from phenylalanine, which is converted by PAL to trans- cinnamate. Trans-cinnamate is hydroxylated by trans-cinnamate 4-monooxygenase to give 4-hydroxycinnamate (i.e, coumarate). Coumarate is condensed with coenzyme-A in the presence of 4-coumarate-CoA ligase: :ATP + 4-coumarate + CoA \rightleftharpoons AMP + diphosphate + 4-coumaroyl-CoA. Enzymes using Coumaroyl-Coenzyme A * Anthocyanin 3-O-glucoside 6''-O-hydroxycinnamoyltransferase * Anthocyanin 5-aromatic acyltransferase * Chalcone synthase * 4-Coumarate-CoA ligase * 6'-Deoxychalcone synthase * Agmatine N4-coumaroyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monocotyledonous
Monocotyledons (), commonly referred to as monocots, ( Lilianae ''sensu'' Chase & Reveal) are grass and grass-like flowering plants (angiosperms), the seeds of which typically contain only one embryonic leaf, or cotyledon. They constitute one of the major groups into which the flowering plants have traditionally been divided; the rest of the flowering plants have two cotyledons and are classified as dicotyledons, or dicots. Monocotyledons have almost always been recognized as a group, but with various taxonomic ranks and under several different names. The APG III system of 2009 recognises a clade called "monocots" but does not assign it to a taxonomic rank. The monocotyledons include about 60,000 species, about a quarter of all angiosperms. The largest family in this group (and in the flowering plants as a whole) by number of species are the orchids (family Orchidaceae), with more than 20,000 species. About half as many species belong to the true grasses (Poaceae), which are econ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |