|
Pattern Recognition Receptors
Pattern recognition receptors (PRRs) play a crucial role in the proper function of the innate immune system. PRRs are germline-encoded host sensors, which detect molecules typical for the pathogens. They are proteins expressed, mainly, by cells of the innate immune system, such as dendritic cells, macrophages, monocytes, neutrophils and epithelial cells, to identify two classes of molecules: pathogen-associated molecular patterns (PAMPs), which are associated with microbial pathogens, and damage-associated molecular patterns (DAMPs), which are associated with components of host's cells that are released during cell damage or death. They are also called primitive pattern recognition receptors because they evolved before other parts of the immune system, particularly before adaptive immunity. PRRs also mediate the initiation of antigen-specific adaptive immune response and release of inflammatory cytokines. The microbe-specific molecules that are recognized by a given PRR are calle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Innate Immune System
The innate, or nonspecific, immune system is one of the two main immunity strategies (the other being the adaptive immune system) in vertebrates. The innate immune system is an older evolutionary defense strategy, relatively speaking, and is the dominant immune system response found in plants, fungi, insects, and primitive multicellular organisms (see Beyond vertebrates).. The major functions of the innate immune system are to: * recruit immune cells to infection sites by producing chemical factors, including chemical mediators called cytokines * activate the complement cascade to identify bacteria, activate cells, and promote clearance of antibody complexes or dead cells * identify and remove foreign substances present in organs, tissues, blood and lymph, by specialized white blood cells * activate the adaptive immune system through antigen presentation * act as a physical and chemical barrier to infectious agents; via physical measures such as skin and chemical measures suc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine Triphosphate
Adenosine triphosphate (ATP) is an organic compound that provides energy to drive many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of life, ATP is often referred to as the "molecular unit of currency" of intracellular energy transfer. When consumed in metabolic processes, it converts either to adenosine diphosphate (ADP) or to adenosine monophosphate (AMP). Other processes regenerate ATP. The human body recycles its own body weight equivalent in ATP each day. It is also a precursor to DNA and RNA, and is used as a coenzyme. From the perspective of biochemistry, ATP is classified as a nucleoside triphosphate, which indicates that it consists of three components: a nitrogenous base ( adenine), the sugar ribose, and the triphosphate. Structure ATP consists of an adenine attached by the 9-nitrogen atom to the 1′ carbon atom of a sugar ( ribose), which in tu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homodimers
In biochemistry, a protein dimer is a macromolecular complex formed by two protein monomers, or single proteins, which are usually non-covalently bound. Many macromolecules, such as proteins or nucleic acids, form dimers. The word ''dimer'' has roots meaning "two parts", '' di-'' + '' -mer''. A protein dimer is a type of protein quaternary structure. A protein homodimer is formed by two identical proteins. A protein heterodimer is formed by two different proteins. Most protein dimers in biochemistry are not connected by covalent bonds. An example of a non-covalent heterodimer is the enzyme reverse transcriptase, which is composed of two different amino acid chains. An exception is dimers that are linked by disulfide bridges such as the homodimeric protein NEMO. Some proteins contain specialized domains to ensure dimerization (dimerization domains) and specificity. The G protein-coupled cannabinoid receptors have the ability to form both homo- and heterodimers with several ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
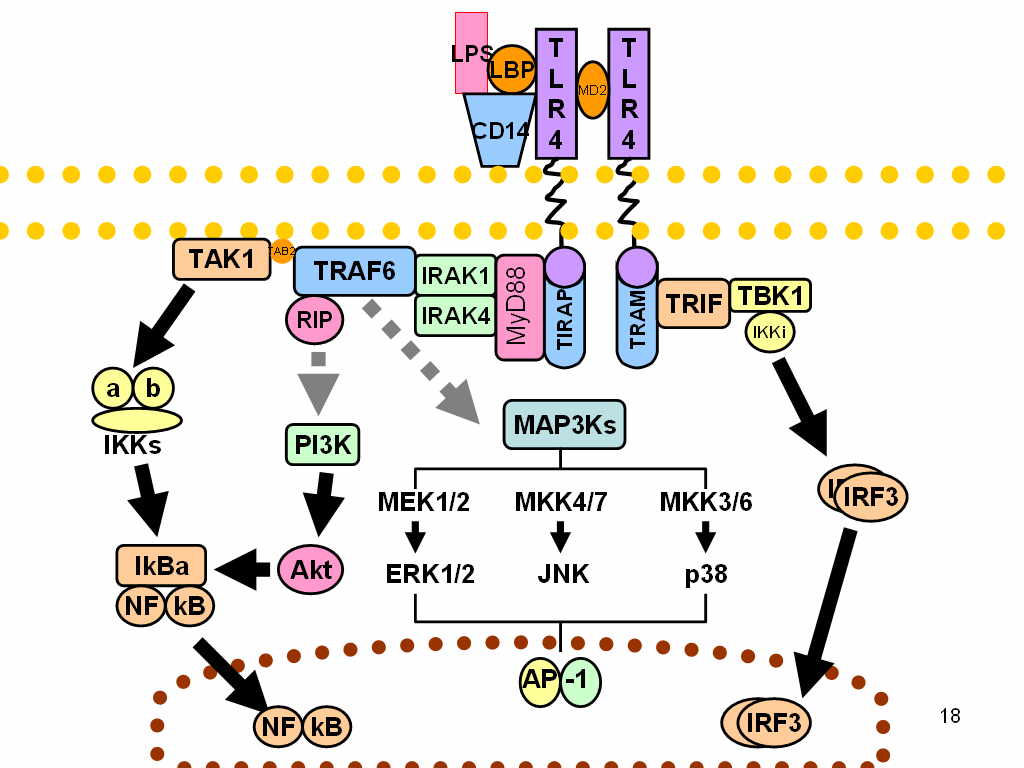
TLR4
Toll-like receptor 4 is a protein that in humans is encoded by the ''TLR4'' gene. TLR4 is a transmembrane protein, member of the toll-like receptor family, which belongs to the pattern recognition receptor (PRR) family. Its activation leads to an intracellular signaling pathway NF-κB and inflammatory cytokine production which is responsible for activating the innate immune system. TLR4 expressing cells are myeloid (erythrocytes, granulocytes, macrophages) rather than lymphoid (T-cells, B-cells, NK cells). Most myeloid cells also express high levels of CD14, which facilitates activation of TLR4 by LPS. It is most well known for recognizing lipopolysaccharide (LPS), a component present in many Gram-negative bacteria (e.g. ''Neisseria'' spp.) and selected Gram-positive bacteria. Its ligands also include several viral proteins, polysaccharide, and a variety of endogenous proteins such as low-density lipoprotein, beta-defensins, and heat shock protein. Palmitic acid and lauric aci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudogene
Pseudogenes are nonfunctional segments of DNA that resemble functional genes. Most arise as superfluous copies of functional genes, either directly by DNA duplication or indirectly by reverse transcription of an mRNA transcript. Pseudogenes are usually identified when genome sequence analysis finds gene-like sequences that lack regulatory sequences needed for transcription or translation, or whose coding sequences are obviously defective due to frameshifts or premature stop codons. Most non-bacterial genomes contain many pseudogenes, often as many as functional genes. This is not surprising, since various biological processes are expected to accidentally create pseudogenes, and there are no specialized mechanisms to remove them from genomes. Eventually pseudogenes may be deleted from their genomes by chance DNA replication or DNA repair errors, or they may accumulate so many mutational changes that they are no longer recognizable as former genes. Analysis of these degenerati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TLR11
Toll-like receptor 11 (TLR11) is a protein that in mice and rats is encoded by the gene ''TLR11'', whereas in humans it is represented by a pseudogene. TLR11 belongs to the toll-like receptor (TLR) family and the interleukin-1 receptor/toll-like receptor superfamily. In mice, TLR11 has been shown to recognise (bacterial) flagellin and (eukaryotic) profilin present on certain microbes, it helps propagate a host immune response. TLR11 plays a fundamental role in both the innate and adaptive immune responses, through the activation of Tumor necrosis factor-alpha, the Interleukin 12 (IL-12) response, and Interferon-gamma (IFN-gamma) secretion. TLR11 mounts an immune response to multiple microbes, including '' Toxoplasma gondii'' (''T. gondii''), '' Salmonella'' species, and uropathogenic ''E. coli'', and likely many other species due to the highly conserved nature of flagellin and profilin. Structure and localization Proteins in the TLR family are pattern recognition receptors w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytokine
Cytokines are a broad and loose category of small proteins (~5–25 kDa) important in cell signaling. Cytokines are peptides and cannot cross the lipid bilayer of cells to enter the cytoplasm. Cytokines have been shown to be involved in autocrine signaling, autocrine, paracrine signaling, paracrine and endocrine signaling as Immunomodulation, immunomodulating agents. Cytokines include chemokines, interferons, interleukins, lymphokines, and tumour necrosis factors, but generally not hormones or growth factors (despite some growth factor#cytokine, overlap in the terminology). Cytokines are produced by a broad range of cells, including immune cells like macrophages, B cell, B lymphocytes, T cell, T lymphocytes and mast cells, as well as Endothelium, endothelial cells, fibroblasts, and various stromal cells; a given cytokine may be produced by more than one type of cell. They act through cell surface receptors and are especially important in the immune system; cytokines modulate the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drosophila Melanogaster
''Drosophila melanogaster'' is a species of fly (the taxonomic order Diptera) in the family Drosophilidae. The species is often referred to as the fruit fly or lesser fruit fly, or less commonly the " vinegar fly" or "pomace fly". Starting with Charles W. Woodworth's 1901 proposal of the use of this species as a model organism, ''D. melanogaster'' continues to be widely used for biological research in genetics, physiology, microbial pathogenesis, and life history evolution. As of 2017, five Nobel Prizes have been awarded to drosophilists for their work using the insect. ''D. melanogaster'' is typically used in research owing to its rapid life cycle, relatively simple genetics with only four pairs of chromosomes, and large number of offspring per generation. It was originally an African species, with all non-African lineages having a common origin. Its geographic range includes all continents, including islands. ''D. melanogaster'' is a common pest in homes, restaurants, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leucine Rich Repeat
A leucine-rich repeat (LRR) is a protein structural motif that forms an α/β horseshoe fold. It is composed of repeating 20–30 amino acid stretches that are unusually rich in the hydrophobic amino acid leucine. These tandem repeats commonly fold together to form a solenoid protein domain, termed leucine-rich repeat domain. Typically, each repeat unit has beta strand- turn- alpha helix structure, and the assembled domain, composed of many such repeats, has a horseshoe shape with an interior parallel beta sheet and an exterior array of helices. One face of the beta sheet and one side of the helix array are exposed to solvent and are therefore dominated by hydrophilic residues. The region between the helices and sheets is the protein's hydrophobic core and is tightly sterically packed with leucine residues. Leucine-rich repeats are frequently involved in the formation of protein–protein interactions. Examples Leucine-rich repeat motifs have been identified in a large nu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toll-like Receptor
Toll-like receptors (TLRs) are a class of proteins that play a key role in the innate immune system. They are single-pass membrane-spanning receptors usually expressed on sentinel cells such as macrophages and dendritic cells, that recognize structurally conserved molecules derived from microbes. Once these microbes have reached physical barriers such as the skin or intestinal tract mucosa, they are recognized by TLRs, which activate immune cell responses. The TLRs include TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9, TLR10, TLR11, TLR12, and TLR13. Humans lack genes for TLR11, TLR12 and TLR13 and mice lack a functional gene for TLR10. TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are located on the cell membrane, whereas TLR3, TLR7, TLR8, and TLR9 are located in intracellular vesicles (because they are sensors of nucleic acids). TLRs received their name from their similarity to the protein coded by the toll gene. Function The ability of the immune system to re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RIG-I-like Receptor
RIG-like receptors (retinoic acid-inducible gene-I-like receptors, RLRs) are a type of intracellular pattern recognition receptor involved in the recognition of viruses by the innate immune system. RIG-I (retinoic-acid inducible gene or DDX58) is the best characterized receptor within the RIG-I like receptor (RLR) family. Together with MDA5 (melanoma differentiation-associated 5) and LGP2 (laboratory of genetics and physiology 2), this family of cytoplasmic pattern recognition receptors (PRRs) are sentinels for intracellular viral RNA that is a product of viral infection. The RLR receptors provide frontline defence against viral infections in most tissues. RLR ligands The RIG-I receptor prefers to bind short (2000 bp), such as the replicative form of picornavirus RNA that is found in picornavirus-infected cells. LGP2 binds to blunt-ended double-stranded RNA of variable length, and also to RNA-bound MDA5 to regulate filament formation. The latter is linked to LGP2's recognition of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NOD-like Receptor
The nucleotide-binding oligomerization domain-like receptors, or NOD-like receptors (NLRs) (also known as nucleotide-binding leucine-rich repeat receptors), are intracellular sensors of pathogen-associated molecular patterns (PAMPs) that enter the cell via phagocytosis or pores, and damage-associated molecular patterns (DAMPs) that are associated with cell stress. They are types of pattern recognition receptors (PRRs), and play key roles in the regulation of innate immune response. NLRs can cooperate with toll-like receptors (TLRs) and regulate inflammatory and apoptotic response. They are found in lymphocytes, macrophages, dendritic cells and also in non-immune cells, for example in epithelium. NLRs are highly conserved through evolution. Their homologs have been discovered in many different animal species (APAF1) and also in the plant kingdom ( disease-resistance R protein). Structure NLRs contain 3 domains – central NACHT (NOD or NBD – nucleotide-binding domain) dom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |