|
Proteolipid
A proteolipid is a protein covalently linked to lipid molecules, which can be fatty acids, isoprenoids or sterols. The process of such a linkage is known as protein lipidation, and falls into the wider category of acylation and post-translational modification. Proteolipids are abundant in brain tissue, and are also present in many other animal and plant tissues. They include ghrelin, a peptide hormone associated with feeding. Many proteolipids have bound fatty acid chains, which often provide an interface for interacting with biological membranes and act as lipidons that direct proteins to specific zones. Proteolipids were discovered serendipitously in 1951 by Jordi Folch Pi and Marjorie Lees while extracting sulfatides from brain lipids. They are not to be confused with lipoproteins, a kind of spherical assembly made up of many molecules of lipids and some apolipoproteins. Structure Depending on the type of fatty acid attached to the protein, a proteolipid can often ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Marjorie Lees
Marjorie Berman Lees (1923–2012) was an American neuroscientist who was emeritus professor of biological chemistry at Harvard Medical School. Her research considered neurobiology and biochemistry. She was the first to identify the Folch-Lees proteolipid. She served as president of the American Society for Neurochemistry in 1983. Early life and education Lees was born in New York City and was educated in the New York Public School System. She attended Hunter High School, where she credited her physics and chemistry teacher with her enthusiasm for science. She was an undergraduate student at Hunter College, where she was introduced to neuroscience and the nervous system of the ''Xenopus''. Lees enrolled in a master's course at the University of Chicago, where she investigated the brains of fish. She was particularly interested in the regions that gave rise to their light-seeking behavior. She obtained her master's towards the end of World War II, and met a soldier returning fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jordi Folch Pi
Jordi Folch Pi (March 25, 1911October 3, 1979) was a Spanish biochemist at Harvard University (McLean Hospital) who was recognized universally as one of the founders of the field of structural chemistry of complex lipids and as a leader in the development of neurochemistry as a distinct discipline within the neurosciences. Early life Folch was born in Barcelona, Spain. His father, Rafel Folch, was a lawyer and a Catalan poet, and his mother, Maria Pi, a teacher. Folch went to high school at the Lycée Français of Barcelona, from which he graduated in 1927. He went on to study medicine, receiving an M.D. degree from the University of Barcelona Medical school in 1932. Early career Folch's clinical training at university included a period as an intern in the surgical clinic of Dr. Antoni Trias and as the sole physician in Almedret, a small Catalan village of 800 people. Folch had the opportunity to study at the Institute of Physiology in Barcelona, an institute dedicated to car ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
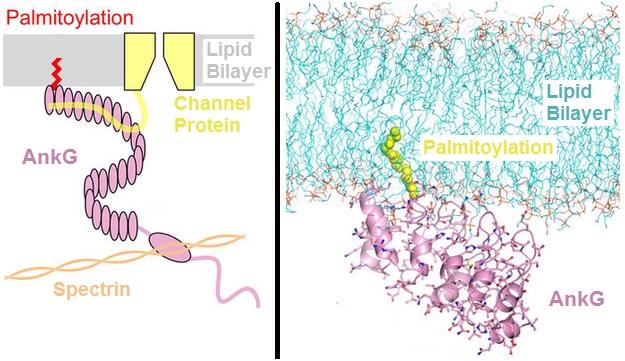
Palmitoyl Group
In molecular biology, palmitoylation is the covalent attachment of fatty acids, such as palmitic acid, to cysteine (''S''-palmitoylation) and less frequently to serine and threonine (''O''-palmitoylation) residues of proteins, which are typically membrane proteins. The precise function of palmitoylation depends on the particular protein being considered. Palmitoylation enhances the hydrophobicity of proteins and contributes to their membrane association. Palmitoylation also appears to play a significant role in subcellular trafficking of proteins between membrane compartments, as well as in modulating protein–protein interactions. In contrast to prenylation and myristoylation, palmitoylation is usually reversible (because the bond between palmitic acid and protein is often a thioester bond). The reverse reaction in mammalian cells is catalyzed by acyl-protein thioesterases (APTs) in the cytosol and palmitoyl protein thioesterases in lysosomes. Because palmitoylation is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipoproteins
A lipoprotein is a biochemical assembly whose primary function is to transport hydrophobic lipid (also known as fat) molecules in water, as in blood plasma or other extracellular fluids. They consist of a triglyceride and cholesterol center, surrounded by a phospholipid outer shell, with the hydrophilic portions oriented outward toward the surrounding water and lipophilic portions oriented inward toward the lipid center. A special kind of protein, called apolipoprotein, is embedded in the outer shell, both stabilising the complex and giving it a functional identity that determines its role. Plasma lipoprotein particles are commonly divided into five main classes, based on size, lipid composition, and apolipoprotein content. They are, in increasing size order: HDL, LDL, IDL, VLDL and chylomicrons. Subgroups of these plasma particles are primary drivers or modulators of atherosclerosis. Many enzymes, transporters, structural proteins, antigens, adhesins, and toxins are somet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ghrelin
Ghrelin (; or lenomorelin, INN) is a hormone primarily produced by enteroendocrine cells of the gastrointestinal tract, especially the stomach, and is often called a "hunger hormone" because it increases the drive to eat. Blood levels of ghrelin are highest before meals when hungry, returning to lower levels after mealtimes. Ghrelin may help prepare for food intake by increasing gastric motility and stimulating the secretion of gastric acid. Ghrelin activates cells in the anterior pituitary gland and hypothalamic arcuate nucleus, including neuropeptide Y neurons that initiate appetite. Ghrelin stimulates brain structures having a specific receptor – the growth hormone secretagogue receptor 1A ( GHSR-1A). Ghrelin also participates in regulation of reward cognition, learning and memory, the sleep-wake cycle, taste sensation, reward behavior, and glucose metabolism. History and name Ghrelin was discovered after the ghrelin receptor (called growth hormone secretag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
S-palmitoylation
In molecular biology, palmitoylation is the covalent attachment of fatty acids, such as palmitic acid, to cysteine (''S''-palmitoylation) and less frequently to serine and threonine (''O''-palmitoylation) residues of proteins, which are typically membrane proteins. The precise function of palmitoylation depends on the particular protein being considered. Palmitoylation enhances the hydrophobicity of proteins and contributes to their membrane association. Palmitoylation also appears to play a significant role in subcellular trafficking of proteins between membrane compartments, as well as in modulating protein–protein interactions. In contrast to prenylation and myristoylation, palmitoylation is usually reversible (because the bond between palmitic acid and protein is often a thioester bond). The reverse reaction in mammalian cells is catalyzed by acyl-protein thioesterases (APTs) in the cytosol and palmitoyl protein thioesterases in lysosomes. Because palmitoylation is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (GGU, GGC, GGA, GGG). Glycine disrupts the formation of alpha-helices in secondary protein structure. Its small side chain causes it to favor random coils instead. Glycine is also an inhibitory neurotransmitter – interference with its release within the spinal cord (such as during a '' Clostridium tetani'' infection) can cause spastic paralysis due to uninhibited muscle contraction. It is the only achiral proteinogenic amino acid. It can fit into both hydrophilic and hydrophobic environments, due to its minimal side chain of only one hydrogen atom. History and etymology Glycine was discovered in 1820 by French chemist Henri Braconnot when he hydrolyzed gelatin by boiling it with sulfuric acid. He originally called it "sugar of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as a nucleophile. Cysteine is chiral, but both D and L-cysteine are found in nature. LCysteine is a protein monomer in all biota, and D-cysteine acts as a signaling molecule in mammalian nervous systems. Cysteine is named after its discovery in urine, which comes from the urinary bladder or cyst, from Ancient Greek, Greek κύστις ''kýstis'', "bladder". The thiol is susceptible to oxidation to give the disulfide bond, disulfide derivative cystine, which serves an important structural role in many proteins. In this case, the symbol Cyx is sometimes used. The deprotonated form can generally be described by the symbol Cym as well. When used as a food additive, cysteine has the E number E920. Cysteine is Genetic code, encoded by the codo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prenylation
Prenylation (also known as isoprenylation or lipidation) is the addition of hydrophobic molecules to a protein or a biomolecule. It is usually assumed that prenyl groups (3-methylbut-2-en-1-yl) facilitate attachment to cell membranes, similar to lipid anchors like the GPI anchor, though direct evidence of this has not been observed. Prenyl groups (also called isoprenyl groups, having one hydrogen atom more than isoprene) have been shown to be important for protein–protein binding through specialized prenyl-binding domains. Protein prenylation Protein prenylation involves the transfer of either a farnesyl or a geranylgeranyl moiety to C-terminal cysteine(s) of the target protein. There are three enzymes that carry out prenylation in the cell, farnesyl transferase, Caax protease and geranylgeranyl transferase I. Farnesylation is a type of prenylation, a post-translational modification of proteins by which an isoprenyl group is added to a cysteine residue. It is an important ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
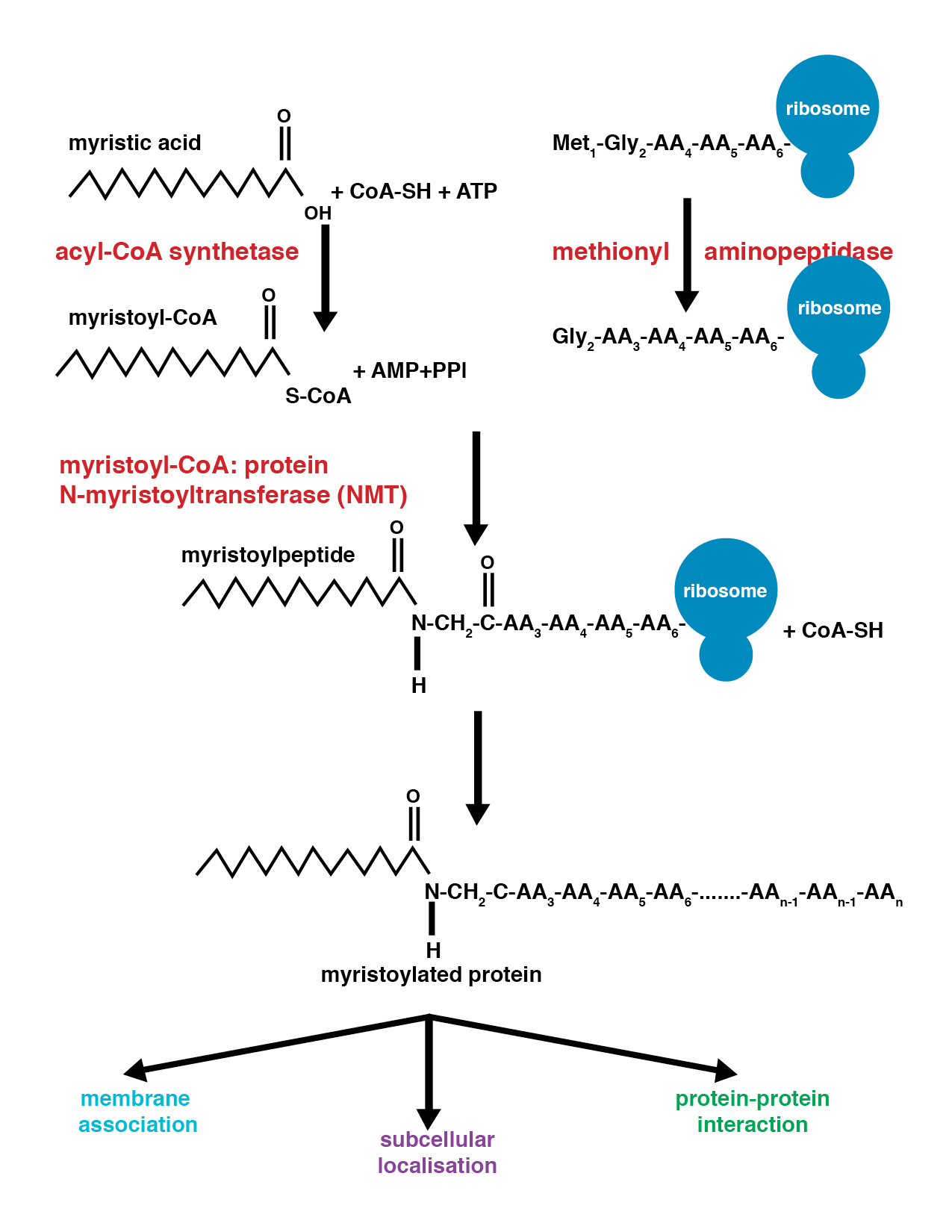
Myristoylation
Myristoylation is a lipidation modification where a myristoyl group, derived from myristic acid, is covalently attached by an amide bond to the alpha-amino group of an ''N''-terminal glycine residue. Myristic acid is a 14-carbon saturated fatty acid (14:0) with the systematic name of ''n''-tetradecanoic acid. This modification can be added either co-translationally or post-translationally. ''N''-myristoyltransferase (NMT) catalyzes the myristic acid addition reaction in the cytoplasm of cells. This lipidation event is the most common type of fatty acylation and is present in many organisms, including animals, plants, fungi, protozoans and viruses. Myristoylation allows for weak protein–protein and protein–lipid interactions and plays an essential role in membrane targeting, protein–protein interactions and functions widely in a variety of signal transduction pathways. Discovery In 1982, Koiti Titani's lab identified an "''N''-terminal blocking group" on the catal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |