|
Prion
A prion () is a Proteinopathy, misfolded protein that induces misfolding in normal variants of the same protein, leading to cellular death. Prions are responsible for prion diseases, known as transmissible spongiform encephalopathy (TSEs), which are fatal and transmissible neurodegenerative diseases affecting both humans and animals. These proteins can misfold sporadically, due to genetic mutations, or by exposure to an already misfolded protein, leading to an abnormal Protein tertiary structure, three-dimensional structure that can propagate misfolding in other proteins. The term ''prion'' comes from "proteinaceous infectious particle". Unlike other infectious agents such as viruses, bacteria, and fungi, prions do not contain nucleic acids (DNA or RNA). Prions are mainly twisted Protein isoform, isoforms of the major prion protein (PrP), a naturally occurring protein with an uncertain function. They are the hypothesized cause of various transmissible spongiform encephalopath ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmissible Spongiform Encephalopathy
Transmissible spongiform encephalopathies (TSEs), also known as prion diseases, are a group of progressive, incurable, and fatal conditions that are associated with the prion hypothesis and affect the brain and nervous system of many animals, including humans, cattle, and sheep. According to the most widespread hypothesis, they are transmitted by prions, though some other data suggest an involvement of a ''Spiroplasma'' infection. Mental and physical abilities deteriorate and many tiny holes appear in the cortex causing it to appear like a sponge when brain tissue obtained at autopsy is examined under a microscope. The disorders cause impairment of brain function which may result in memory loss, personality changes, and abnormal or impaired movement which worsen over time. TSEs of humans include Creutzfeldt–Jakob disease, Gerstmann–Sträussler–Scheinker syndrome, fatal familial insomnia, and kuru, as well as the recently discovered variably protease-sensitive prionopat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
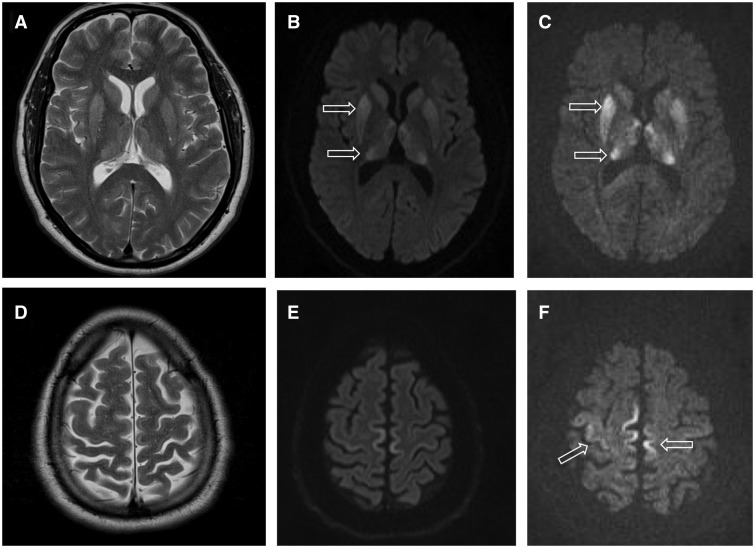
Creutzfeldt–Jakob Disease
Creutzfeldt–Jakob disease (CJD) is an incurable, always fatal neurodegenerative disease belonging to the transmissible spongiform encephalopathy (TSE) group. Early symptoms include memory problems, behavioral changes, poor coordination, visual disturbances and auditory disturbances. Later symptoms include dementia, involuntary movements, blindness, deafness, weakness, and coma. About 70% of sufferers die within a year of diagnosis. The name "Creutzfeldt–Jakob disease" was introduced by Walther Spielmeyer in 1922, after the German neurologists Hans Gerhard Creutzfeldt and Alfons Maria Jakob. CJD is caused by abnormal folding of a protein known as a prion. Infectious prions are misfolded proteins that can cause normally folded proteins to also become misfolded. About 85% of cases of CJD occur for unknown reasons, while about 7.5% of cases are inherited in an autosomal dominant manner. Exposure to brain or spinal tissue from an infected person may also result in spread. Ther ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
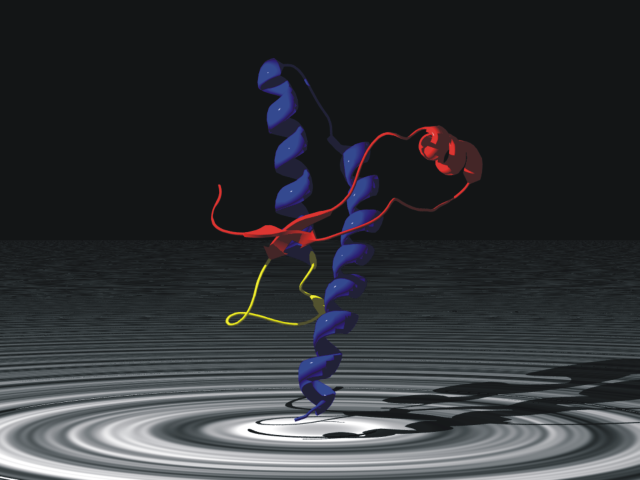
Major Prion Protein
The major prion protein (PrP) is encoded in the human body by the ''PRNP'' gene also known as CD230 (cluster of differentiation 230). Expression of the protein is most predominant in the nervous system but occurs in many other tissues throughout the body. The protein can exist in multiple isoforms: the normal PrPC form, and the protease-resistant form designated PrPRes such as the disease-causing PrPSc (scrapie) and an isoform located in mitochondria. The misfolded version PrPSc is associated with a variety of cognitive disorders and neurodegenerative diseases such as in animals: ovine scrapie, bovine spongiform encephalopathy (BSE, mad cow disease), feline spongiform encephalopathy, transmissible mink encephalopathy (TME), exotic ungulate encephalopathy, chronic wasting disease (CWD) which affects deer; and in humans: Creutzfeldt–Jakob disease (CJD), fatal familial insomnia (FFI), Gerstmann–Sträussler–Scheinker syndrome (GSS), kuru, and variant Creutzfeldt–Jak ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scrapie
Scrapie () is a fatal, degenerative disease affecting the nervous systems of sheep and goats. It is one of several transmissible spongiform encephalopathies (TSEs), and as such it is thought to be caused by a prion. Scrapie has been known since at least 1732 and does not appear to be transmissible to humans. However, it has been found to be experimentally transmissible to humanised transgenic mice and non-human primates. The name scrapie is derived from one of the clinical signs of the condition, wherein affected animals will compulsively scrape off their fleeces against rocks, trees or fences. The disease apparently causes an itching sensation in the animals. Other clinical signs include excessive lip smacking, altered gaits and convulsive collapse. Scrapie is infectious and transmissible among conspecifics, so one of the most common ways to contain it (since it is incurable) is to quarantine and kill those affected. However, scrapie tends to persist in flocks and can also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chronic Wasting Disease
Chronic wasting disease (CWD), sometimes called zombie deer disease, is a transmissible spongiform encephalopathy (TSE) affecting deer. TSEs are a family of diseases thought to be caused by misfolded proteins called prions and include similar diseases such as BSE (mad cow disease) in cattle, Creutzfeldt–Jakob disease (CJD) in humans, and scrapie in sheep. Natural infection causing CWD affects members of the deer family. In the United States, CWD affects mule deer, white-tailed deer, red deer, sika deer, elk, bison, antelope, caribou, and moose. The transmission of CWD to other species such as squirrel monkeys and humanized mice has been observed in experimental settings. In 1967, CWD was first identified in mule deer at a government research facility in northern Colorado, United States. It was initially recognized as a clinical "wasting" syndrome and then in 1978, it was identified more specifically as a TSE disease. Since then, CWD has been found in free-rang ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bovine Spongiform Encephalopathy
Bovine spongiform encephalopathy (BSE), commonly known as mad cow disease, is an incurable and always fatal neurodegenerative disease of cattle. Symptoms include abnormal behavior, trouble walking, and weight loss. Later in the course of the disease, the cow becomes unable to function normally. There is conflicting information about the time between infection and onset of symptoms. In 2002, the World Health Organization suggested it to be approximately four to five years. Time from onset of symptoms to death is generally weeks to months. Spread to humans is believed to result in variant Creutzfeldt–Jakob disease (vCJD). , a total of 233 cases of vCJD had been reported globally. BSE is thought to be due to an infection by a misfolded protein, known as a prion. Cattle are believed to have been infected by being fed meat-and-bone meal that contained either the remains of cattle who spontaneously developed the disease or scrapie-infected sheep products. The United Kingdo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prion (bird)
The prions () or whalebirds are small petrels in the genera ''Pachyptila'' and '' Halobaena''. They form one of the four groups within the Procellariidae along with the gadfly petrels, shearwaters and fulmarine petrels. The name comes from the Greek ', meaning "saw", a reference of the serrated edges of the birds' saw-like bill. They are found in the Southern Ocean and breed on a number of subantarctic islands. Prions grow long, and have blue-grey upper parts and white underparts. Three species of prion have flattened bills with a fringe of lamellae that act as strainers for zooplankton.Maynard, B. J. (2003) All prions are marine and feed on small crustacea such as copepods, ostracods, decapods, and krill, as well as some fish such as myctophids and nototheniids. List of species * ''Pachyptila'' ** ''Pachyptila turtur'', fairy prion ** ''Pachyptila belcheri'', slender-billed prion ** ''Pachyptila crassirostris'', fulmar prion ** ''Pachyptila vittata'', broad-billed pri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteinopathy
In medicine, proteinopathy ( 'pref''. protein -pathy 'suff''. disease proteinopathies ''pl''.; proteinopathic ''adj''), or proteopathy, protein conformational disorder, or protein misfolding disease, is a class of diseases in which certain proteins become structurally abnormal, and thereby disrupt the function of cells, tissues and organs of the body. Often the proteins fail to fold into their normal configuration; in this misfolded state, the proteins can become toxic in some way (a toxic gain-of-function) or they can lose their normal function. The proteinopathies include such diseases as Creutzfeldt–Jakob disease (and a variant associated with mad cow disease) and other prion diseases, Alzheimer's disease, Parkinson's disease, amyloidosis, multiple system atrophy, and a wide range of other disorders. The term ''proteopathy'' was first proposed in 2000 by Lary Walker and Harry LeVine. The concept of proteopathy can trace its origins to the mid-19th century, wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
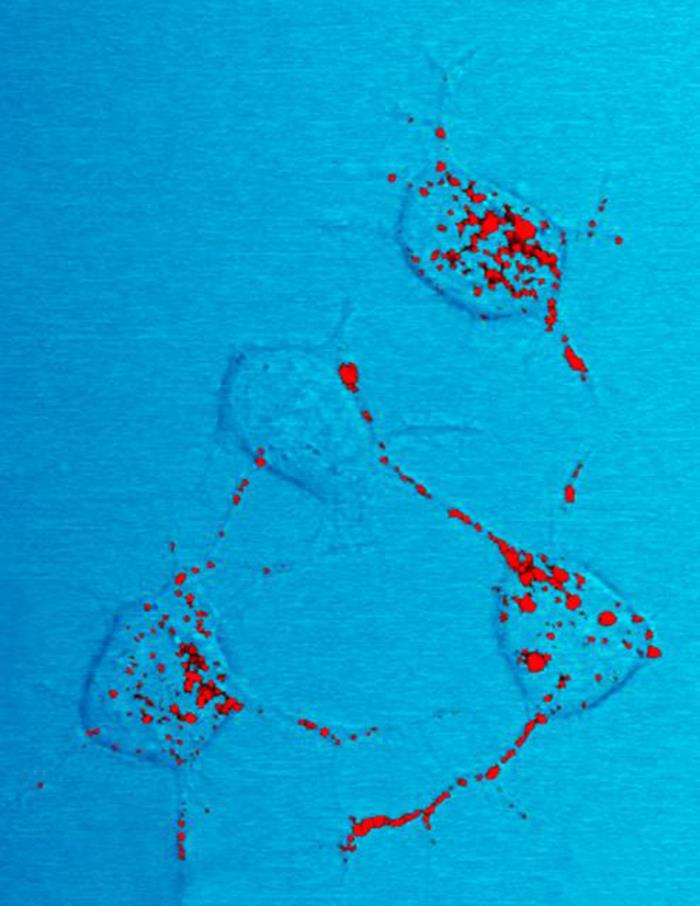
Amyloids
Amyloids are aggregates of proteins characterised by a fibrillar morphology of typically 7–13 nm in diameter, a β-sheet secondary structure (known as cross-β) and ability to be stained by particular dyes, such as Congo red. In the human body, amyloids have been linked to the development of various diseases. Pathogenic amyloids form when previously healthy proteins lose their normal structure and physiological functions ( misfolding) and form fibrous deposits within and around cells. These protein misfolding and deposition processes disrupt the healthy function of tissues and organs. Such amyloids have been associated with (but not necessarily as the cause of) more than 50 human diseases, known as amyloidosis, and may play a role in some neurodegenerative diseases. Some of these diseases are mainly sporadic and only a few cases are familial. Others are only familial. Some result from medical treatment. Prions are an infectious form of amyloids that can act as a template ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neurodegenerative Disease
A neurodegenerative disease is caused by the progressive loss of neurons, in the process known as neurodegeneration. Neuronal damage may also ultimately result in their death. Neurodegenerative diseases include amyotrophic lateral sclerosis, multiple sclerosis, Parkinson's disease, Alzheimer's disease, Huntington's disease, multiple system atrophy, tauopathies, and prion diseases. Neurodegeneration can be found in the brain at many different levels of neuronal circuitry, ranging from molecular to systemic. Because there is no known way to reverse the progressive degeneration of neurons, these diseases are considered to be incurable; however research has shown that the two major contributing factors to neurodegeneration are oxidative stress and inflammation. Biomedical research has revealed many similarities between these diseases at the subcellular level, including atypical protein assemblies (like proteinopathy) and induced cell death. These similarities suggest that therap ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-synuclein
Alpha-synuclein (aSyn) is a protein that in humans is encoded by the ''SNCA'' gene. It is a neuronal protein involved in the regulation of synaptic vesicle trafficking and the release of neurotransmitters. Alpha-synuclein is abundant in the brain, with smaller amounts present in the heart, muscles, and other tissues. Within the brain, it is primarily localized to the axon terminals of presynaptic neurons. There, it interacts with phospholipids and other proteins. Presynaptic terminals release neurotransmitters from specialized compartments called synaptic vesicles, a process essential for neuronal communication and normal brain function. In Parkinson's disease and related synucleinopathies, abnormal, insoluble forms of alpha-synuclein accumulate within neurons as inclusion bodies, inclusions known as Lewy body, Lewy bodies. Mutations in the ''SNCA'' gene are linked to familial forms of Parkinson's disease. During the process of seeded nucleation, alpha-synuclein adopts a cross-be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Multiple System Atrophy
Multiple system atrophy (MSA) is a rare neurodegenerative disorder characterized by tremors, slow movement, muscle rigidity, postural instability (collectively known as parkinsonism), autonomic dysfunction and ataxia. This is caused by progressive degeneration of neurons in several parts of the brain including the basal ganglia, inferior olivary nucleus, and cerebellum. MSA was first described in 1960 by Milton Shy and Glen Drager and was then known as Shy–Drager syndrome. Many people affected by MSA experience dysfunction of the autonomic nervous system, which commonly manifests as orthostatic hypotension, impotence, loss of sweating, dry mouth and urinary retention and incontinence. Palsy of the vocal cords is an important and sometimes initial clinical manifestation of the disorder. A prion of the alpha-synuclein protein within affected neurons may cause MSA. About 55% of MSA cases occur in men, with those affected first showing symptoms at the age of 50–60 ye ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |