|
Power-law Fluid
In continuum mechanics, a power-law fluid, or the Ostwald–de Waele relationship, is a type of generalized Newtonian fluid. This mathematical relationship is useful because of its simplicity, but only approximately describes the behaviour of a real non-Newtonian fluid. Power-law fluids can be subdivided into three different types of fluids based on the value of their flow behaviour index: pseudoplastic, Newtonian fluid, and dilatant. A first-order fluid is another name for a power-law fluid with exponential dependence of viscosity on temperature. As a Newtonian fluid in a circular pipe give a quadratic velocity profile, a power-law fluid will result in a power-law velocity profile. Description In continuum mechanics, a power-law fluid, or the Ostwald–de Waele relationship, is a type of generalized Newtonian fluid (time-independent non-Newtonian fluid) for which the shear stress, , is given by :\tau = K \left( \frac \right)^n where: * is the ''flow consistency index'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Continuum Mechanics
Continuum mechanics is a branch of mechanics that deals with the deformation of and transmission of forces through materials modeled as a ''continuous medium'' (also called a ''continuum'') rather than as discrete particles. Continuum mechanics deals with ''deformable bodies'', as opposed to rigid bodies. A continuum model assumes that the substance of the object completely fills the space it occupies. While ignoring the fact that matter is made of atoms, this provides a sufficiently accurate description of matter on length scales much greater than that of inter-atomic distances. The concept of a continuous medium allows for intuitive analysis of bulk matter by using differential equations that describe the behavior of such matter according to physical laws, such as mass conservation, momentum conservation, and energy conservation. Information about the specific material is expressed in constitutive relationships. Continuum mechanics treats the physical properties of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Armand De Waele
Armand Michel A. de Waele (17 November 1887 – December 1966) was a British chemist, noted for his contributions to rheology, and after whom the Ostwald–de Waele relationship for non-Newtonian fluids is named.Ostwald called it the de Waele-Ostwald equation: ''Kolloid Zeitschrift'' (1929) 47 (2) 176-187 De Waele was born in Islington, London, in 1887, the son of a Belgian father and French mother. He held dual nationality until the age of 21, when he chose to be a British rather than Belgian. He obtained a BSc from the Regent Street Polytechnic then worked in the paint and linoleum industries. In 1914, the same year was conscripted into the Royal Flying Corps.''Chemistry and Industry'', May 13, 1967, page 777 Obituary; Armand de Waele (1887-1966) That same year, he married a Frenchwoman, Jeanne Thérèse Duvivier (1892–1971), in Staines. They had two sons, John and Peter After the First World War, he joined Gestetner as Chief Research Chemist, where he remained ti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
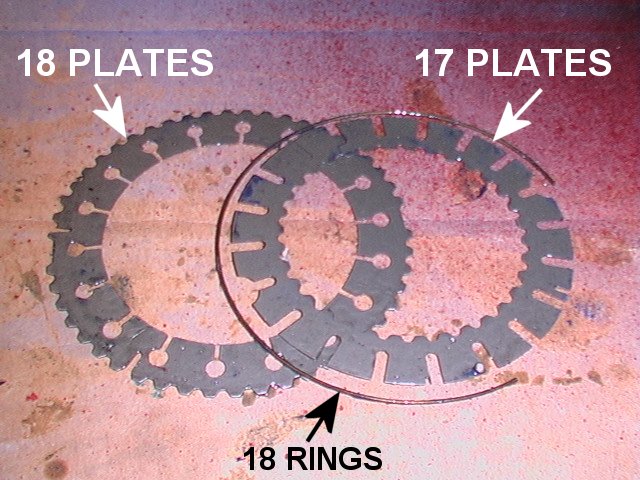
Viscous Coupling
A viscous coupling is a mechanical device which transfers torque and rotation by the medium of a viscous fluid. Design Rotary viscous couplings with interleaved, perforated plates and filled with viscous fluids are used in automotive systems to transmit torque. The device consists of a number of circular plates with tabs or perforations, fitted very close to each other in a sealed drum. Alternate plates are connected to a driving shaft at one end of the assembly and a driven shaft at the other end. The drum is filled with a dilatant fluid, often silicone-based, to about 80% by volume. When the two sets of plates are rotating in unison, the fluid stays cool and remains liquid. When the plates start rotating at different speeds, the shear effect of the tabs or perforations on the fluid will cause it to heat and become nearly solid because the viscosity Viscosity is a measure of a fluid's rate-dependent drag (physics), resistance to a change in shape or to movement of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aqueous Solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, also known as sodium chloride (NaCl), in water would be represented as . The word ''aqueous'' (which comes from ''aqua'') means pertaining to, related to, similar to, or dissolved in, water. As water is an excellent solvent and is also naturally abundant, it is a ubiquitous solvent in chemistry. Since water is frequently used as the solvent in experiments, the word solution refers to an aqueous solution, unless the solvent is specified. A ''non-aqueous solution'' is a solution in which the solvent is a liquid, but is not water. Characteristics Substances that are ''hydrophobic'' ('water-fearing') do not dissolve well in water, whereas those that are '' hydrophilic'' ('water-friendly') do. An example of a hydrophilic substance is sodium chloride. In an aqueous solution the hydrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food energy or organic micronutrients. Its chemical formula, , indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. In liquid form, is also called "water" at standard temperature and pressure. Because Earth's environment is relatively close to water's triple point, water exists on Earth as a solid, a liquid, and a gas. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycerine
Glycerol () is a simple triol compound. It is a colorless, odorless, sweet-tasting, viscous liquid. The glycerol backbone is found in lipids known as glycerides. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature. Modern use of the word glycerine (alternatively spelled glycerin) refers to commercial preparations of less than 100% purity, typically 95% glycerol. Structure Although achiral, glycerol is prochiral with respect to reactions of one of the two primary alcohols. Thus, in substituted derivatives, the stereospecific numbering labels the molecule with a ''sn''- prefix before the stem name of the molecule. Production Natural sources Glycerol is generally obtained from plant and animal sources where it occurs in triglycerides, esters of glycerol with long-chain carboxylic acids. The hydrolysis, saponificatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corn Syrup
Corn syrup is a food syrup that is made from the starch of corn/maize and contains varying amounts of sugars: glucose, maltose and higher oligosaccharides, depending on the grade. Corn syrup is used in foods to soften Mouthfeel, texture, add volume, prevent crystallization of sugar, and enhance flavor. Most table syrups are typically based with corn syrup. It can be processed into high-fructose corn syrup (HFCS) by using the enzyme xylose isomerase, D-xylose isomerase to convert a large proportion of its glucose into sweeter fructose. The more general term glucose syrup is often used synonymously with corn syrup, since glucose syrup in the United States is most commonly made from corn starch. Technically, glucose syrup is any liquid starch hydrolysis, hydrolysate of mono-, di-, and higher-saccharides and can be made from any source of starch: wheat, tapioca and potatoes are the most common other sources. Commercial preparation Historically, corn syrup was produced by combining ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compound (chemistry), compounds, produces unique physical property, physical properties including toughness, high rubber elasticity, elasticity, viscoelasticity, and a tendency to form Amorphous solid, amorphous and crystallization of polymers, semicrystalline structures rath ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solution (chemistry)
In chemistry, a solution is defined by IUPAC as "A liquid or solid phase containing more than one substance, when for convenience one (or more) substance, which is called the solvent, is treated differently from the other substances, which are called solutes. When, as is often but not necessarily the case, the sum of the mole fractions of solutes is small compared with unity, the solution is called a dilute solution. A superscript attached to the ∞ symbol for a property of a solution denotes the property in the limit of infinite dilution." One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term " aqueous solution" is used when one of the solvents is water. Types ''Homogeneous'' means that the components of the mixture form a single phase. ''Heterogeneous'' means that the components of the mixture are of different phase. The properties of the mixture (such as concentration, temp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apparent Viscosity
In fluid mechanics, apparent viscosity (sometimes denoted ) is the shear stress applied to a fluid divided by the shear rate: :\eta = \frac For a Newtonian fluid, the apparent viscosity is constant, and equal to the Newtonian viscosity of the fluid, but for non-Newtonian fluids, the apparent viscosity depends on the shear rate. Apparent viscosity has the SI derived unit Pa·s ( Pascal-second), but the centipoise is frequently used in practice: (1 mPa·s = 1 cP). Application A single viscosity measurement at a constant speed in a typical viscometer is a measurement of the instrument viscosity of a fluid (not the apparent viscosity). In the case of non-Newtonian fluids, measurement of apparent viscosity without knowledge of the shear rate is of limited value: the measurement cannot be compared to other measurements if the speed and geometry of the two instruments is not identical. An apparent viscosity that is reported without the shear rate or information about the instrument ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dilatant
A dilatant (, ) (also termed shear thickening) material is one in which viscosity increases with the rate of shear strain. Such a ''shear thickening fluid'', also known by the initialism ''STF'', is an example of a non-Newtonian fluid. This behaviour is usually not observed in pure materials, but can occur in suspensions. A dilatant is a non-Newtonian fluid where the shear viscosity increases with applied shear stress. This behavior is only one type of deviation from Newton's law of viscosity, and it is controlled by such factors as particle size, shape, and distribution. The properties of these suspensions depend on Hamaker theory and Van der Waals forces and can be stabilized electrostatically or sterically. Shear thickening behavior occurs when a colloidal suspension transitions from a stable state to a state of flocculation. A large portion of the properties of these systems are due to the surface chemistry of particles in dispersion, known as colloids. This can read ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudoplastic
In rheology, shear thinning is the non-Newtonian behavior of fluids whose viscosity decreases under shear strain. It is sometimes considered synonymous for pseudo-plastic behaviour, and is usually defined as excluding time-dependent effects, such as thixotropy. Shear thinning is the most common type of non-Newtonian behavior of fluids and is seen in many industrial and everyday applications. Although shear thinning is generally not observed in pure liquids with low molecular mass or ideal solutions of small molecules like sucrose or sodium chloride, it is often observed in polymer solutions and molten polymers, as well as complex fluids and suspensions like ketchup, whipped cream, blood, paint, and nail polish. Theories behind shear-thinning behaviour Though the exact cause of shear thinning is not fully understood, it is widely regarded to be the effect of small structural changes within the fluid, such that microscale geometries within the fluid rearrange to facilitat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |