|
Polysulfane
A polysulfane is a chemical compound of formula , where ''n'' > 1 (although disulfane () is sometimes excluded). Compounds containing 2 – 8 sulfur atoms have been isolated, longer chain compounds have been detected, but only in solution.R. Steudel "Inorganic Polysulfanes H2S''n'' with ''n'' > 1" in Elemental Sulfur and Sulfur-Rich Compounds II (Topics in Current Chemistry) 2003, Volume 231, pp 99–125. is colourless, higher members are yellow with the colour increasing with the sulfur content. In the chemical literature the term polysulfanes is sometimes used for compounds containing , e.g. organic polysulfanes . Structures Polysulfanes consist of unbranched chains of sulfur atoms terminated with hydrogen atoms. The branched isomer of tetrasulfane , in which the fourth sulfur is bonded to the central sulfur, would be described as trithiosulfurous acid, . Computations suggests that it is less stable than the linear isomer . The S-S-S angles approach 90° in trisulfane and hig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulfur Dichloride
Disulfur dichloride (or disulphur dichloride by the British English spelling) is the inorganic compound of sulfur and chlorine with the Chemical formula, formula . It is an amber oily liquid. Sometimes, this compound is incorrectly named ''sulfur monochloride'' (or ''sulphur monochloride'' by the British English spelling), the name implied by its empirical formula SCl. has the structure implied by the formula , wherein the dihedral angle between the and planes is 85.2°. This structure is referred to as Conformational isomerism, gauche, and is akin to that for Hydrogen peroxide, . A rare isomer of is (thiothionyl chloride); this isomer forms transiently when is exposed to UV-radiation (see thiosulfoxides). Synthesis, basic properties, reactions Disulfur dichloride is a yellow liquid that fumes in moist air due to reaction with water: : It is produced by partial chlorination of elemental sulfur. The reaction proceeds at usable rates at room temperature. In the laboratory, c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
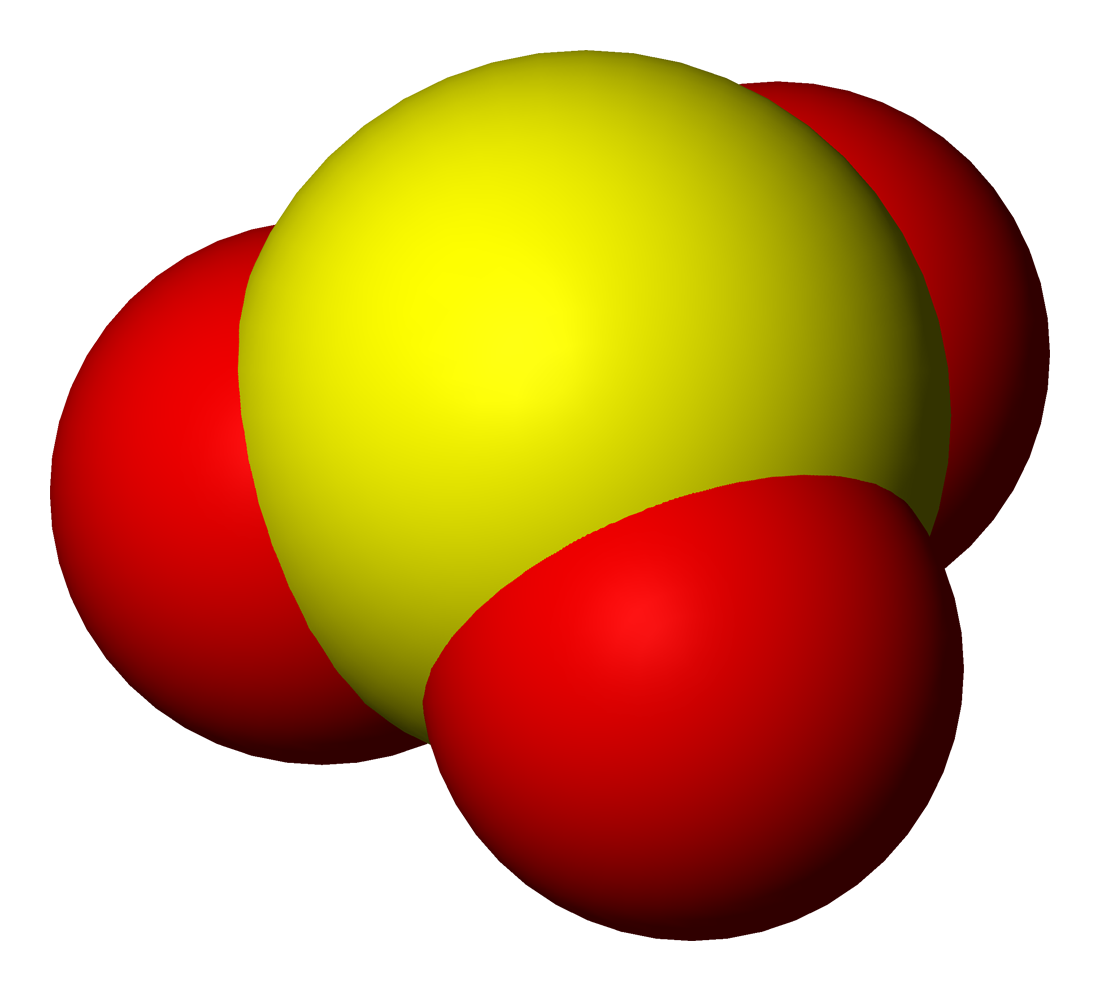
Hydrogen Disulfide
Hydrogen disulfide is the inorganic compound with the formula . This hydrogen chalcogenide is a pale yellow volatile liquid with a camphor-like odor. It decomposes readily to hydrogen sulfide () and elemental sulfur. Structure The connection of atoms in the hydrogen disulfide molecule is . The structure of hydrogen disulfide is similar to that of hydrogen peroxide, with C2 point group symmetry. Both molecules are distinctly nonplanar. The dihedral angle between the and planes is 90.6°, compared with 111.5° in . The bond angle is 92°, close to 90° for unhybridized divalent sulfur. Synthesis Hydrogen disulfide can be synthesised by cracking polysulfanes () according to this idealized equation: : The main impurity is trisulfane (). The precursor polysulfane is produced by the reaction of hydrochloric acid with aqueous sodium polysulfide. The polysulfane precipitates as an oil. Reactions Upon contact with water or alcohols, hydrogen disulfide readily decomposes under ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allotropes Of Sulfur
The element sulfur exists as many allotropes. In number of allotropes, sulfur is second only to Allotropes of carbon, carbon.#Greenwood, Greenwood, 652 In addition to the allotropes, each allotrope often exists in Polymorphism (materials science), polymorphs (different crystal structures of the same covalently bonded Sn molecules) delineated by Greek prefixes (α, β, etc.). Furthermore, because elemental sulfur has been an item of commerce for centuries, its various forms are given traditional names. Early workers identified some forms that have later proved to be single or mixtures of allotropes. Some forms have been named for their appearance, e.g. "mother of pearl sulfur", or alternatively named for a chemist who was pre-eminent in identifying them, e.g. "Muthmann's sulfur I" or "Engel's sulfur".#Steudel, Steudel, 17 The most commonly encountered form of sulfur is the orthorhombic polymorph of , which adopts a puckered ring – or "crown" – structure. Two other polymorphs a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulfane
Hydrogen disulfide is the inorganic compound with the formula . This hydrogen chalcogenide is a pale yellow volatile liquid with a camphor-like odor. It decomposes readily to hydrogen sulfide () and elemental sulfur. Structure The connection of atoms in the hydrogen disulfide molecule is . The structure of hydrogen disulfide is similar to that of hydrogen peroxide, with C2 point group symmetry. Both molecules are distinctly nonplanar. The dihedral angle between the and planes is 90.6°, compared with 111.5° in . The bond angle is 92°, close to 90° for unhybridized divalent sulfur. Synthesis Hydrogen disulfide can be synthesised by cracking polysulfanes () according to this idealized equation: : The main impurity is trisulfane (). The precursor polysulfane is produced by the reaction of hydrochloric acid with aqueous sodium polysulfide. The polysulfane precipitates as an oil. Reactions Upon contact with water or alcohols, hydrogen disulfide readily decomposes under ambien ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polysulfide
Polysulfides are a class of chemical compounds derived from anionic chains of sulfur atoms. There are two main classes of polysulfides: inorganic and organic. The inorganic polysulfides have the general formula . These anions are the conjugate bases of polysulfanes . Organic polysulfides generally have the formulae , where R is an alkyl or aryl group. Polysulfide salts and complexes The alkali metal polysulfides arise by treatment of a solution of the sulfide with elemental sulfur, e.g. sodium sulfide to sodium polysulfide: In some cases, these anions have been obtained as organic salts, which are soluble in organic solvents. The energy released in the reaction of sodium and elemental sulfur is the basis of battery technology. The sodium–sulfur battery and the lithium–sulfur battery require high temperatures to maintain liquid polysulfide and -conductive membranes that are unreactive toward sodium, sulfur, and sodium sulfide. Polysulfides are ligands in coordination ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trisulfane
Trisulfane is the inorganic compound with the formula . It is a pale yellow volatile liquid with a camphor-like odor. It decomposes readily to hydrogen sulfide () and elemental sulfur. It is produced by distillation of the polysulfane oil obtained by acidification of polysulfide Polysulfides are a class of chemical compounds derived from anionic chains of sulfur atoms. There are two main classes of polysulfides: inorganic and organic. The inorganic polysulfides have the general formula . These anions are the conjugate bas ... salts.R. Steudel "Inorganic Polysulfanes H2Sn with n > 1" in Elemental Sulfur and Sulfur-Rich Compounds II (Topics in Current Chemistry) 2003, Volume 231, pp 99-125. References Hydrogen compounds Inorganic sulfur compounds {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vapour Pressure
Vapor pressure or equilibrium vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The equilibrium vapor pressure is an indication of a liquid's thermodynamic tendency to evaporate. It relates to the balance of particles escaping from the liquid (or solid) in equilibrium with those in a coexisting vapor phase. A substance with a high vapor pressure at normal temperatures is often referred to as '' volatile''. The pressure exhibited by vapor present above a liquid surface is known as vapor pressure. As the temperature of a liquid increases, the attractive interactions between liquid molecules become less significant in comparison to the entropy of those molecules in the gas phase, increasing the vapor pressure. Thus, liquids with strong intermolecular interactions are likely to have smaller vapor pressures, with the reverse true for weaker interactions. The vapor p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Extrapolated
In mathematics, extrapolation is a type of estimation, beyond the original observation range, of the value of a variable on the basis of its relationship with another variable. It is similar to interpolation, which produces estimates between known observations, but extrapolation is subject to greater uncertainty and a higher risk of producing meaningless results. Extrapolation may also mean extension of a method, assuming similar methods will be applicable. Extrapolation may also apply to human experience to project, extend, or expand known experience into an area not known or previously experienced. By doing so, one makes an assumption of the unknownExtrapolation entry at Merriam–Webster (for example, a driver ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist Carl Wilhelm Scheele is credited with having discovered the chemical composition of purified hydrogen sulfide in 1777. Hydrogen sulfide is toxic to humans and most other animals by inhibiting cellular respiration in a manner similar to hydrogen cyanide. When it is inhaled or its salts are ingested in high amounts, damage to organs occurs rapidly with symptoms ranging from breathing difficulties to convulsions and death. Despite this, the human body produces small amounts of this sulfide and its mineral salts, and uses it as a signalling molecule. Hydrogen sulfide is often produced from the microbial breakdown of organic matter in the absence of oxygen, such as in swamps and sewers; this process is commonly known as anaerobic digestio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanide
In chemistry, cyanide () is an inorganic chemical compound that contains a functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom. Ionic cyanides contain the cyanide anion . This anion is extremely poisonous. Soluble cyanide salts such as sodium cyanide (NaCN), potassium cyanide (KCN) and tetraethylammonium cyanide () are highly toxic. Covalent cyanides contain the group, and are usually called nitriles if the group is linked by a single covalent bond to carbon atom. For example, in acetonitrile , the cyanide group is bonded to methyl . In tetracyanomethane , four cyano groups are bonded to carbon. Although nitriles generally do not release cyanide ions, the cyanohydrins do and are thus toxic. The cyano group may be covalently bonded to atoms different than carbon, e.g., in cyanogen azide , phosphorus tricyanide and trimethylsilyl cyanide . Hydrogen cyanide, or , is a highly volatile toxic liquid tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfite
Sulfites or sulphites are compounds that contain the sulfite ion (systematic name: sulfate(IV) ion), . The sulfite ion is the conjugate base of bisulfite. Although its acid (sulfurous acid) is elusive, its salts are widely used. Sulfites are substances that naturally occur in some foods and the human body. They are also used as regulated food additives. When in food or drink, sulfites are often lumped together with sulfur dioxide.SeREGULATION (EU) No 1169/2011 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL/ref> Structure The structure of the sulfite anion can be described with three equivalent resonance structures. In each resonance structure, the sulfur atom is double-bonded to one oxygen atom with a formal charge of zero (neutral), and sulfur is singly bonded to the other two oxygen atoms, which each carry a formal charge of −1, together accounting for the −2 charge on the anion. There is also a non-bonded lone pair on the sulfur, so the structure predicted by VSEPR ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name since it does not contain any words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called '' empirical formulae'', which use letters and numbers indicating the numerical ''proportions'' of atoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |