|
Pine Resin
A resin is a solid or highly viscous liquid that can be converted into a polymer. Resins may be biological or synthetic in origin, but are typically harvested from plants. Resins are mixtures of organic compounds, predominantly terpenes. Common resins include amber, hashish, frankincense, myrrh and the animal-derived resin, shellac. Resins are used in varnishes, adhesives, food additives, incenses and perfumes. Resins protect plants from insects and pathogens, and are secreted in response to injury. Resins repel herbivores, insects, and pathogens, while the volatile phenolic compounds may attract benefactors such as predators of insects that attack the plant. Composition Most plant resins are composed of terpenes. Specific components are alpha-pinene, beta-pinene, delta-3 carene, and sabinene, the monocyclic terpenes limonene and terpinolene, and smaller amounts of the tricyclic sesquiterpenes, longifolene, caryophyllene, and delta-cadinene. Some resins also contain a hi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Natural Phenol
In biochemistry, naturally occurring phenols are natural products containing at least one phenol functional group. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants. Classification Various classification schemes can be applied. A commonly used scheme is based on the number of carbons and was devised by Jeffrey Harborne and Simmonds in 1964 and published in 1980: C6-C7-C6 Diarylheptanoids are not included in this Harborne classification. They can also be classified on the basis of their number of phenol groups. They can therefore be called ''simple phenols'' or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Balm Of Gilead
Balm of Gilead was a rare perfume used medicinally that was mentioned in the Hebrew Bible and named for the region of Gilead, where it was produced. The expression stems from William Tyndale's language in the King James Bible of 1611 and has come to signify a universal cure in figurative speech. The tree or shrub producing the balm is commonly identified as '' Commiphora gileadensis''. However, some botanical scholars have concluded that the actual source was a terebinth tree in the genus '' Pistacia''.Groom (1981) History Hebrew Bible In the Bible, balsam is designated by various names: (''bosem''), (''bessem''), (''tẓawree''), נָטָף (''nawt-off''), which all differ from the terms used in rabbinic literature. After having cast Joseph into a pit, his brothers noticed a caravan on its way from Gilead to Egypt, "with their camels bearing spicery, and balm, and myrrh" ( Gen. ). When Jacob dispatched his embassy into Egypt, his present to the unknown ruler included " ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rosin
Rosin (), also known as colophony or Greek pitch (), is a resinous material obtained from pine trees and other plants, mostly conifers. The primary components of rosin are diterpenoids, i.e., C20 carboxylic acids. Rosin consists mainly of resin acids, especially abietic acid. Rosin often appears as a semi-transparent, brittle substance that ranges in color from yellow to black and melts at stove-top temperatures. In addition to industrial applications such as in varnishes, adhesives, and sealing wax, rosin is used with string instruments on the bow hair to enhance its ability to grip and sound the strings, and it provides grip in various sports and activities. Rosin also serves as an ingredient in medicinal and pharmaceutical formulations and can cause contact dermatitis or occupational asthma in sensitive individuals. It is an FDA approved food additive. The name "colophony" originates from , Latin for "resin from Colophon" (), an ancient Ionic city. Properties R ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Resin Acid
Resin acid refers to any of several related carboxylic acids found in tree resins. Nearly all resin acids have the same basic skeleton: three fused rings having the empirical formula C19H29COOH. Resin acids occur in nature as tacky, yellowish gums consisting of several compounds. They are water-insoluble. A common resin acid is abietic acid. Resin acids are used to produce soaps for diverse applications, but their use is being displaced increasingly by synthetic acids such as 2-ethylhexanoic acid or petroleum-derived naphthenic acids. Botanical analysis Resin acids are protectants and wood preservatives that are produced by parenchymatous epithelial cells that surround the resin ducts in trees from temperate coniferous forests. The resin acids are formed when two-carbon and three-carbon molecules couple with isoprene building units to form monoterpenes (volatile), sesquiterpenes (volatile), and diterpenes (nonvolatile) structures. Pines contain numerous vertical and radial r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadinene
Cadinenes are a group of isomeric hydrocarbons that occur in a wide variety of essential oil-producing plants. The name is derived from that of the Cade juniper (''Juniperus oxycedrus'' L.), the wood of which yields an oil from which cadinene isomers were first isolated. Chemically, the cadinenes are bicyclic sesquiterpenes. The term ''cadinene'' has sometimes also been used in a broad sense to refer to any sesquiterpene with the so-called cadalane (4-isopropyl-1,6-dimethyldecahydronaphthalene) carbon skeleton. Because of the large number of known double-bond and stereochemical isomers, this class of compounds has been subdivided into four subclasses based on the relative stereochemistry at the isopropyl group and the two bridgehead carbon atoms. The name ''cadinene'' is now properly used only for the first subclass below, which includes the compounds originally isolated from cade oil. Only one enantiomer In chemistry, an enantiomer (Help:IPA/English, /ɪˈnænti.əmə ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caryophyllene
Caryophyllene (), more formally (−)-β-caryophyllene (BCP), is a natural bicyclic sesquiterpene that occurs widely in nature. Caryophyllene is notable for having a cyclobutane ring, as well as a ''trans''-double bond in a 9-membered ring, both rarities in nature. Production Caryophyllene can be produced synthetically, but it is invariably obtained from natural sources because it is widespread. It is a constituent of many essential oils, especially clove oil, the oil from the stems and flowers of ''Syzygium aromaticum'' (cloves), the essential oil of ''Cannabis sativa'', copaiba, rosemary, and hops. It is usually found as a mixture with isocaryophyllene (the ''cis'' double bond isomer) and humulene, α-humulene (obsolete name: α-caryophyllene), a ring-opened isomer. Caryophyllene is one of the chemical compounds that contributes to the odor, aroma of black pepper. Basic research β-Caryophyllene is under basic research for its potential action as an agonist of the cannab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Longifolene
Longifolene is a common sesquiterpene. It is an oily liquid hydrocarbon found primarily in the high-boiling fraction of certain pine resins. The name is derived from that of a pine species from which the compound was isolated. It is a tricyclic chiral molecule. The enantiomer commonly found in pines and other higher plants exhibits a positive optical rotation of +42.73°. The other enantiomer (optical rotation −42.73°) is found in small amounts in certain fungi and liverworts. Occurrence Terpentine obtained from ''Pinus longifolia'' (obsolete name for ''Pinus roxburghii'' Sarg.) contains as much as 20% of longifolene. Longifolene is also one of two most abundant aroma constituents of lapsang souchong tea, because the tea is smoked over pinewood fires. Biosynthesis The biosynthesis of longifolene begins with farnesyl diphosphate (1) (also called farnesyl pyrophosphate) by means of a cationic polycyclization cascade. Loss of the pyrophosphate group and cyclization by the dist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sesquiterpene
Sesquiterpenes are a class of terpenes that consist of three isoprene units and often have the molecular formula C15H24. Like monoterpenes, sesquiterpenes may be cyclic or contain rings, including many combinations. Biochemical modifications such as oxidation or rearrangement produce the related sesquiterpenoids. It is estimated (2006) that 3000 sesquiterpenes have been identified. Biosynthesis and examples The reaction of geranyl pyrophosphate with isopentenyl pyrophosphate results in the 15-carbon farnesyl pyrophosphate (FPP), which is an intermediate in the biosynthesis of sesquiterpenes such as farnesene. Cyclic sesquiterpenes are more common than cyclic monoterpenes because of the increased chain length and additional double bond in the sesquiterpene precursors. In addition to common six-membered ring systems such as the ones found in zingiberene and bisacurone, cyclization of one end of the chain to the other end can lead to macrocyclic rings such as humulene. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terpinolene
The terpinenes are a group of isomeric hydrocarbons that are classified as monoterpenes. They each have the same molecular formula and carbon framework, but they differ in the position of carbon-carbon double bonds. α-Terpinene has been isolated from cardamom and marjoram oils, and from other natural sources. β-Terpinene has no known natural source but has been prepared from sabinene. γ-Terpinene and δ-terpinene (also known as terpinolene) have been isolated from a variety of plant sources. They are all colorless liquids with a turpentine-like odor. Production and uses α-Terpinene is produced industrially by acid-catalyzed rearrangement of α-pinene. It has perfume and flavoring properties but is mainly used to confer pleasant odor to industrial fluids. Hydrogenation gives the saturated derivative P-Menthane, ''p''-menthane. Biosynthesis of α-terpinene The biosynthesis of α-terpinene and other terpenoids starts with the isomerization of geranyl pyrophosphate to lina ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Limonene
Limonene () is a colorless liquid aliphatic hydrocarbon classified as a cyclic monoterpene, and is the major component in the essential oil of citrus fruit peels. The (+)-isomer, occurring more commonly in nature as the fragrance of oranges, is a flavoring agent in food manufacturing. It is also used in chemical synthesis as a precursor to carvone and as a renewables-based solvent in cleaning products. The less common (−)-isomer has a piny, turpentine-like odor, and is found in the edible parts of such plants as caraway, dill, and bergamot orange plants. Limonene takes its name from Italian ''limone'' ("lemon"). Limonene is a chiral molecule, and biological sources produce one enantiomer: the principal industrial source, citrus fruit, contains (+)-limonene (''d''-limonene), which is the (''R'')-enantiomer. (+)-Limonene is obtained commercially from citrus fruits through two primary methods: centrifugal separation or steam distillation. In plants (+)-Limonene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
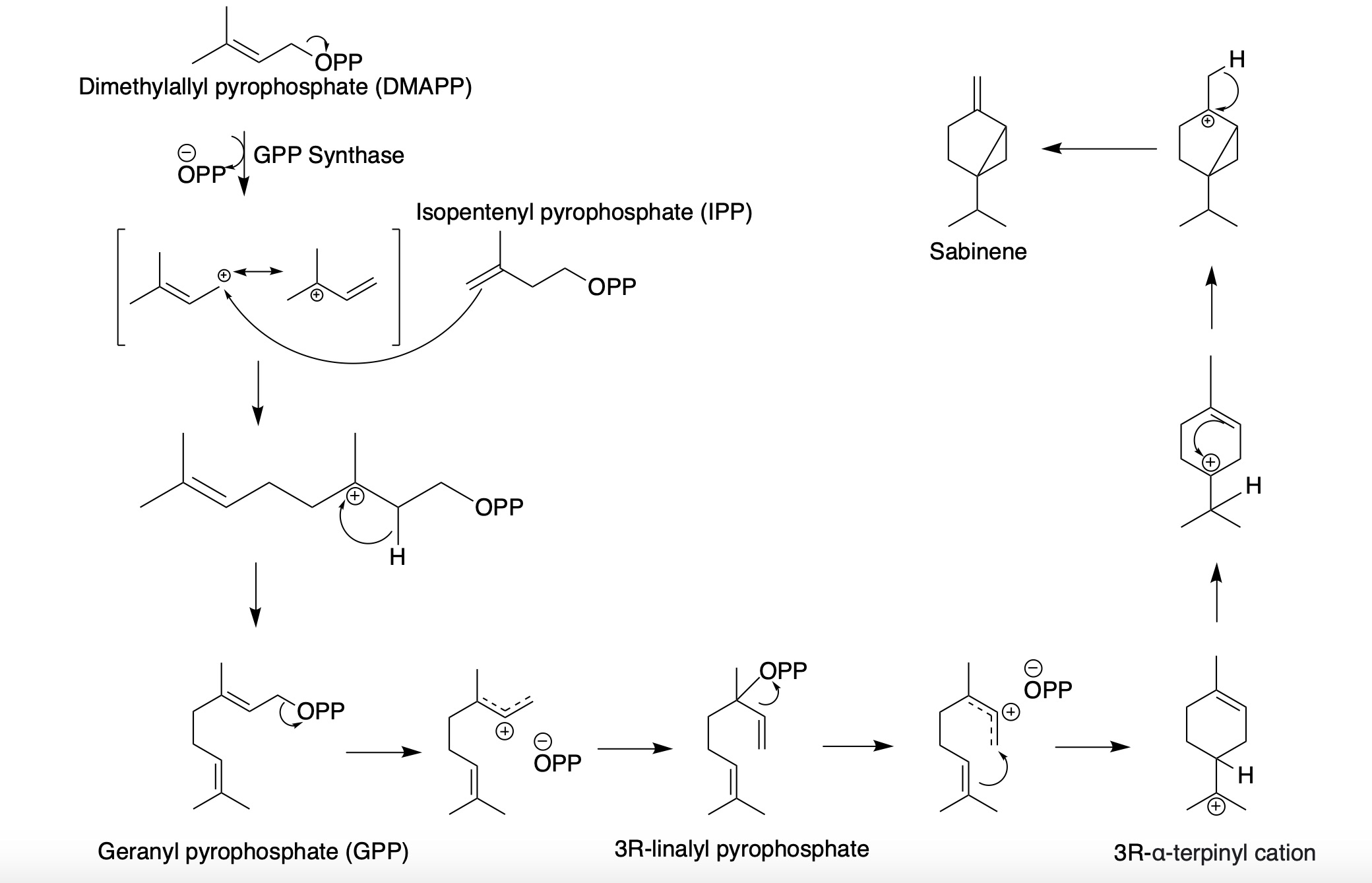
Sabinene
Sabinene is a natural bicyclic monoterpene with the molecular formula C10H16. It is isolated from the essential oils of a variety of plants including Marjoram, holm oak (''Quercus ilex'') and Norway spruce (''Picea abies''). It has a strained ring system with a cyclopentane ring fused to a cyclopropane ring. Sabinene is one of the chemical compounds that contributes to the spiciness of black pepper and is a major constituent of carrot seed oil. It also occurs in tea tree oil at a low concentration. It is also present in the essential oil obtained from nutmeg, '' Laurus nobilis'', and '' Clausena anisata''. Biosynthesis Sabinene, a bicyclic monoterpene, is present in the (+) and (-) enantiomer In chemistry, an enantiomer (Help:IPA/English, /ɪˈnænti.əmər, ɛ-, -oʊ-/ Help:Pronunciation respelling key, ''ih-NAN-tee-ə-mər''), also known as an optical isomer, antipode, or optical antipode, is one of a pair of molecular entities whi ...s. It is biosynthesized from the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |