|
Peroxidases
Peroxidases or peroxide reductases ( EC numberbr>1.11.1.x are a large group of enzymes which play a role in various biological processes. They are named after the fact that they commonly break up peroxides, and should not be confused with other enzymes that ''produce'' peroxide, which are often oxidases. Functionality Peroxidases typically catalyze a reaction of the form: :ROOR' + \overset + 2H+ -> ce + R'OH Optimal substrates For many of these enzymes the optimal substrate is hydrogen peroxide, but others are more active with organic hydroperoxides such as lipid peroxides. Peroxidases can contain a heme cofactor in their active sites, or alternately redox-active cysteine or selenocysteine residues. The nature of the electron donor is very dependent on the structure of the enzyme. * For example, horseradish peroxidase can use a variety of organic compounds as electron donors and acceptors. Horseradish peroxidase has an accessible active site, and many compounds can reach t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haloperoxidase
Haloperoxidases are peroxidases that are able to mediate the oxidation of halides by hydrogen peroxide. Both halides and hydrogen peroxide are widely available in the environment. Mechanistic and thermodynamic considerations Halogenations of organic compounds by free halogens () is generally favorable process. It is practiced industrially on a big scale for example. In nature, however, free halogens do not exist in appreciable amounts. The combination of hydrogen peroxide, which is widely produced by aerobic life, and halide anions provides the equivalent of . The oxidation of these anions by hydrogen peroxide is slow in the absence of enzymes. These enzymes are called haloperoxidases. The reaction that they catalyze is: : From the perspective of thermodynamics, the Nernst equation confirms that hydrogen peroxide can oxidize chloride (E°= 1.36 V), bromide (E°= 1.09 V), and iodide (E°= 0.536 V) from a thermodynamic perspective under natural conditions, i.e., a temperature ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
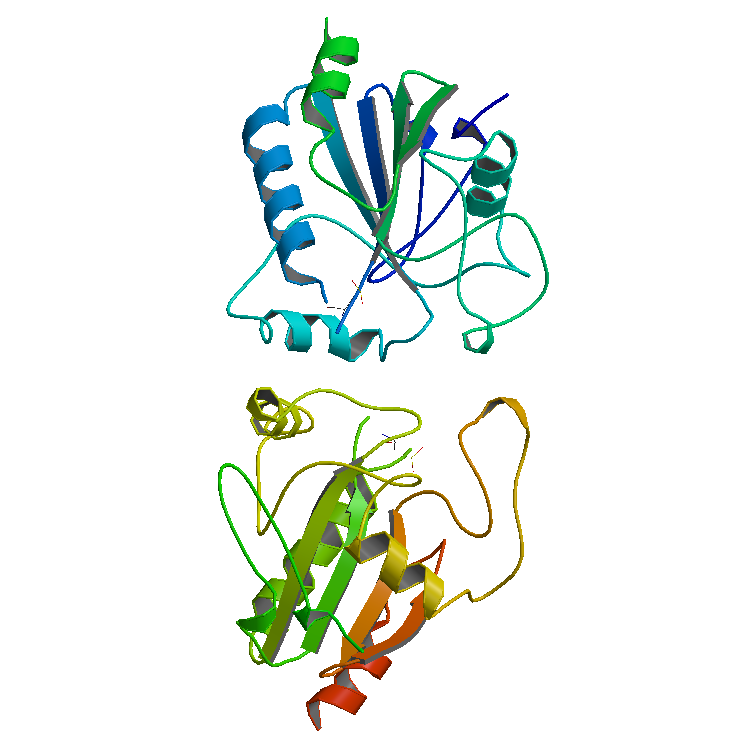
Animal Heme-dependent Peroxidases
Animal heme-dependent peroxidases is a family of peroxidases. Peroxidases are found in bacteria, fungi, plants and animals. On the basis of sequence similarity, a number of animal heme peroxidases can be categorized as members of a superfamily: myeloperoxidase (MPO); eosinophil peroxidase (EPO); lactoperoxidase (LPO); thyroid peroxidase (TPO); prostaglandin H synthase (PGHS); and peroxidasin. Function Myeloperoxidase (MPO) plays a major role in the oxygen-dependent microbicidal system of neutrophils. EPO from eosinophilic granulocytes participates in immunological reactions, and potentiates tumor necrosis factor (TNF) production and hydrogen peroxide release by human monocyte-derived macrophages. MPO (and possibly EPO) primarily use Cl−ions and H2O2 to form hypochlorous acid (HOCl), which can effectively kill bacteria or parasites. In secreted fluids, LPO catalyses the oxidation of thiocyanate ions (SCN−) by H2O2, producing the weak oxidizing agent hypothiocyanite (OSCN−), wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GPX2 (gene)
Glutathione peroxidase 2 is an enzyme that in humans is encoded by the ''GPX2'' gene. This gene is a member of the glutathione peroxidase family encoding a selenium-dependent glutathione peroxidase that is one of two isoenzymes responsible for the majority of the glutathione-dependent hydrogen peroxide-reducing activity in the epithelium of the gastrointestinal tract. Studies in knockout mice indicate that mRNA expression levels respond to luminal microflora, suggesting a role of the ileal glutathione peroxidases in preventing inflammation in the GI tract. The antioxidant enzyme glutathione peroxidase 2 (Gpx2) is one out of eight known glutathione peroxidases (Gpx1-8) in humans. Mammalian Gpx1, GPx2 (this protein), Gpx3, and Gpx4 have been shown to be selenium-containing enzymes, whereas Gpx6 is a selenoprotein in humans with cysteine-containing homologues in rodents. In selenoproteins, the 21st amino acid selenocysteine is inserted in the nascent polypeptide chain during the pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome C Peroxidase
Cytochrome ''c'' peroxidase, or CCP, is a water-soluble heme-containing enzyme of the peroxidase family that takes reducing equivalents from cytochrome ''c'' and reduces hydrogen peroxide to water: :CCP + H2O2 + 2 ferrocytochrome ''c'' + 2H+ → CCP + 2H2O + 2 ferricytochrome ''c'' CCP can be derived from aerobically grown yeast strains and can be isolated in both native and recombinant forms with high yield from ''Saccharomyces cerevisiae.'' The enzyme’s primary function is to eliminate toxic radical molecules produced by the cell which are harmful to biological systems. It works to maintain low concentration levels of hydrogen peroxide, which is generated by the organism naturally through incomplete oxygen reduction. When glucose levels in fast growing yeast strains are exhausted, the cells turn to respiration which raises the concentration of mitochondrial H2O2. In addition to its peroxidase activity, it acts as a sensor and a signaling molecule to exogenous H2O2, which acti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GPX1
Glutathione peroxidase 1, also known as GPx1, is an enzyme that in humans is encoded by the ''GPX1'' gene on chromosome 3. This gene encodes a member of the glutathione peroxidase family. Glutathione peroxidase functions in the detoxification of hydrogen peroxide, and is one of the most important antioxidant enzymes in humans. Structure This gene encodes a member of the glutathione peroxidase family, consisting of eight known glutathione peroxidases (GPx1-8) in humans. Mammalian Gpx1 (this gene), Gpx2, Gpx3, and Gpx4 have been shown to be selenium-containing enzymes, whereas Gpx6 is a selenoprotein in humans with cysteine-containing homologues in rodents. In selenoproteins, the 21st amino acid selenocysteine is inserted in the nascent polypeptide chain during the process of translational recoding of the UGA stop codon. In addition to the UGA-codon, a cis-acting element in the mRNA, called SECIS, binds SBP2 to recruit other proteins, such as eukaryotic elongation factor seleno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NADH Peroxidase
In enzymology, a NADH peroxidase () is an enzyme that catalysis, catalyzes the chemical reaction :NADH + H+ + H2O2 \rightleftharpoons NAD+ + 2 H2O The presumed function of NADH peroxidase is to inactivate H2O2 generated within the cell, for example by glycerol-3-phosphate oxidase during glycerol metabolism or dismutation of superoxide, before the H2O2 causes damage to essential cellular components. The 3 substrate (biochemistry), substrates of this enzyme are nicotinamide adenine dinucleotide, NADH, hydrogen ion, H+, and hydrogen peroxide, H2O2, whereas its two product (chemistry), products are nicotinamide adenine dinucleotide, NAD+ and water, H2O. It employs one cofactor (biochemistry), cofactor, flavin adenine dinucleotide, FAD, however no discrete FADH2 intermediate has been observed. This enzyme belongs to the family of oxidoreductases, specifically those acting on a peroxide as acceptor (peroxidases). The List of enzymes, systematic name of this enzyme class is NADH:hydrog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manganese Peroxidase
In enzymology, a manganese peroxidase () is an enzyme that catalyzes the chemical reaction :2 Mn(II) + 2 H+ + H2O2 \rightleftharpoons 2 Mn(III) + 2 H2O The 3 substrates of this enzyme are Mn(II), H+, and H2O2, whereas its two products are Mn(III) and H2O. This enzyme belongs to the family of oxidoreductases, to be specific those acting on a peroxide as acceptor (peroxidases). The systematic name of this enzyme class is Mn(II):hydrogen-peroxide oxidoreductase. Other names in common use include peroxidase-M2, and Mn-dependent (NADH-oxidizing) peroxidase. It employs one cofactor, heme. This enzyme needs Ca2+ for activity. White rot fungi secrete this enzyme to aid lignin degradation. Discovery and characterization Manganese peroxidase (commonly referred to as MnP) was discovered in 1985 simultaneously by the research groups of Michael H. Gold and Ronald Crawford in the fungus ''Phanerochaete chrysosporium''. The protein was genetically sequenced in ''P. chrysoporium'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanadium Bromoperoxidase
Vanadium bromoperoxidases are a kind of enzymes called haloperoxidases. Its primary function is to remove hydrogen peroxide which is produced during photosynthesis Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ... from in or around the cell. By producing hypobromous acid (HOBr) a secondary reaction with dissolved organic matter, what results is the bromination of organic compounds that are associated with the defense of the organism. These enzymes produce the bulk of natural organobromine compounds in the world. Vanadium bromoperoxidases are one of the few classes of enzymes that requires vanadium. The active site features a vanadium oxide center attached to the protein via one histidine side chain and a collection of hydrogen bonds to the oxide ligands. Occurrence and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxiredoxin
Peroxiredoxins (Prxs, ; HGNC root symbol ''PRDX'') are a ubiquitous family of antioxidant enzymes that also control cytokine-induced peroxide levels and thereby mediate signal transduction in mammalian cells. The family members in humans are PRDX1, PRDX2, PRDX3, PRDX4, PRDX5, and PRDX6. The physiological importance of peroxiredoxins is indicated by their relative abundance (one of the most abundant proteins in erythrocytes after hemoglobin is peroxiredoxin 2). Their function is the reduction of peroxides, specifically hydrogen peroxide, alkyl hydroperoxides, and peroxynitrite. Classification Prxs were historically divided into three (mechanistic) classes: typical 2-Cys Prxs, atypical 2-Cys Prxs, and 1-Cys Prxs. The designation of "1-Cys" and "2-Cys" Prxs was introduced in 1994 as it was noticed that, among the 22 Prx sequences known at the time, only one Cys residue was absolutely conserved; this is the residue now recognized as the (required) peroxidatic cysteine, CP. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutathione Peroxidase
Glutathione peroxidase (GPx) () is the general name of an enzyme family with peroxidase activity whose main biological role is to protect the organism from oxidative damage. The biochemical function of glutathione peroxidase is to reduce lipid hydroperoxides to their corresponding alcohols and to reduce free hydrogen peroxide to water. Glutathione peroxidase was discovered in 1957 by Gordon C. Mills. Reaction The main reaction that glutathione peroxidase catalyzes is: : 2GSH + H2O2 → GS–SG + 2H2O where GSH represents reduced monomeric glutathione, and GS–SG represents glutathione disulfide. The mechanism involves oxidation of the selenol of a selenocysteine residue by hydrogen peroxide. This process gives the derivative with a selenenic acid (RSeOH) group. The selenenic acid is then converted back to the selenol by a two step process that begins with reaction with GSH to form the GS-SeR and water. A second GSH molecule reduces the GS-SeR intermediate back to t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalase
Catalase is a common enzyme found in nearly all living organisms exposed to oxygen (such as bacteria, plants, and animals) which catalyzes the decomposition of hydrogen peroxide to water and oxygen. It is a very important enzyme in protecting the cell from oxidative damage by reactive oxygen species (ROS). Catalase has one of the highest turnover numbers of all enzymes; one catalase molecule can convert millions of hydrogen peroxide molecules to water and oxygen each second. Catalase is a tetramer of four polypeptide chains, each over 500 amino acids long. It contains four iron-containing heme groups that allow the enzyme to react with hydrogen peroxide. The optimum pH for human catalase is approximately 7, and has a fairly broad maximum: the rate of reaction does not change appreciably between pH 6.8 and 7.5. The pH optimum for other catalases varies between 4 and 11 depending on the species. The optimum temperature also varies by species. Structure Human catalase for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haem Peroxidase
Haem peroxidases (or heme peroxidases) are haem-containing enzymes that use hydrogen peroxide Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscosity, viscous than Properties of water, water. It is used as an oxidizer, bleaching agent, and antiseptic, usua ... as the electron acceptor to catalyse a number of oxidative reactions. Most haem peroxidases follow the reaction scheme: : Fe3+ + H2O2 \rightleftharpoons e4+=O' (Compound I) + H2O : e4+=O' + substrate --> e4+=O (Compound II) + oxidized substrate : e4+=O + substrate --> Fe3+ + H2O + oxidized substrate In this mechanism, the enzyme reacts with one equivalent of H2O2 to give e4+=O' (compound I). This is a two-electron oxidation/reduction reaction in which H2O2 is reduced to water, and the enzyme is oxidized. One oxidizing equivalent resides on iron, giving the oxyferryl intermediate, and in many peroxidases the porphyrin (R) is oxidi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |