|
Open-label Study
An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. In particular, both the researchers and participants know which treatment is being administered. This contrasts with a double-blinded trial, where information is withheld both from the researchers and the participants to reduce bias. Open-label trials may be appropriate for comparing two similar treatments to determine which is most effective, such as a comparison of different prescription anticoagulants, or possible relief from symptoms of some disorders when a placebo A placebo ( ) can be roughly defined as a sham medical treatment. Common placebos include inert tablets (like sugar pills), inert injections (like saline), sham surgery, and other procedures. Placebos are used in randomized clinical trials ... is given. An open-label trial may still be randomized. Open-label trials may also be uncontrolled (without a placebo group), with all par ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trial
Clinical trials are prospective biomedical or behavioral research studies on human subject research, human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, pharmaceutical drug, drugs, medical nutrition therapy, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received institutional review board, health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted. Depending on product type and development stage, investigators initially enroll volunteers or patients into small Pilot experiment, pi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blinded Experiment
In a blind or blinded experiment, information which may influence the participants of the experiment is withheld until after the experiment is complete. Good blinding can reduce or eliminate experimental biases that arise from a participants' expectations, observer's effect on the participants, observer bias, confirmation bias, and other sources. A blind can be imposed on any participant of an experiment, including subjects, researchers, technicians, data analysts, and evaluators. In some cases, while blinding would be useful, it is impossible or unethical. For example, it is not possible to blind a patient to their treatment in a physical therapy intervention. A good clinical protocol ensures that blinding is as effective as possible within ethical and practical constraints. During the course of an experiment, a participant becomes unblinded if they deduce or otherwise obtain information that has been masked to them. For example, a patient who experiences a side effect may corr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prescription Drug
A prescription drug (also prescription medication, prescription medicine or prescription-only medication) is a pharmaceutical drug that is permitted to be dispensed only to those with a medical prescription. In contrast, over-the-counter drugs can be obtained without a prescription. The reason for this difference in substance control is the potential scope of misuse, from drug abuse to practising medicine without a license and without sufficient education. Different jurisdictions have different definitions of what constitutes a prescription drug. In North America, , usually printed as "Rx", is used as an abbreviation of the word "prescription". It is a contraction of the Latin word "''recipe''" (an imperative form of "recipere") meaning "take". Prescription drugs are often dispensed together with a monograph (in Europe, a Patient Information Leaflet or PIL) that gives detailed information about the drug. The use of prescription drugs has been increasing since the 1960s. Regul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anticoagulant
An anticoagulant, commonly known as a blood thinner, is a chemical substance that prevents or reduces the coagulation of blood, prolonging the clotting time. Some occur naturally in blood-eating animals, such as leeches and mosquitoes, which help keep the bite area unclotted long enough for the animal to obtain blood. As a class of medications, anticoagulants are used in therapy for thrombotic disorders. Oral anticoagulants (OACs) are taken by many people in pill or tablet form, and various intravenous anticoagulant dosage forms are used in hospitals. Some anticoagulants are used in medical equipment, such as sample tubes, blood transfusion bags, heart–lung machines, and dialysis equipment. One of the first anticoagulants, warfarin, was initially approved as a rodenticide. Anticoagulants are closely related to antiplatelet drugs and thrombolytic drugs by manipulating the various pathways of blood coagulation. Specifically, antiplatelet drugs inhibit platelet agg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Symptom
Signs and symptoms are diagnostic indications of an illness, injury, or condition. Signs are objective and externally observable; symptoms are a person's reported subjective experiences. A sign for example may be a higher or lower temperature than normal, raised or lowered blood pressure or an abnormality showing on a medical scan. A symptom is something out of the ordinary that is experienced by an individual such as feeling feverish, a headache or other pains in the body, which occur as the body's immune system fights off an infection. Signs and symptoms Signs A medical sign is an objective observable indication of a disease, injury, or medical condition that may be detected during a physical examination. These signs may be visible, such as a rash or bruise, or otherwise detectable such as by using a stethoscope or taking blood pressure. Medical signs, along with symptoms, help in forming a diagnosis. Some examples of signs are nail clubbing of either the fingernail ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Placebo
A placebo ( ) can be roughly defined as a sham medical treatment. Common placebos include inert tablets (like sugar pills), inert injections (like saline), sham surgery, and other procedures. Placebos are used in randomized clinical trials to test the efficacy of medical treatments. In a placebo-controlled trial, any change in the control group is known as the ''placebo response'', and the difference between this and the result of no treatment is the ''placebo effect''. Placebos in clinical trials should ideally be indistinguishable from so-called verum treatments under investigation, except for the latter's particular hypothesized medicinal effect. This is to shield test participants (with their consent) from knowing who is getting the placebo and who is getting the treatment under test, as patients' and clinicians' expectations of efficacy can influence results. The idea of a placebo effect was discussed in 18th century psychology, but became more prominent in the 20th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
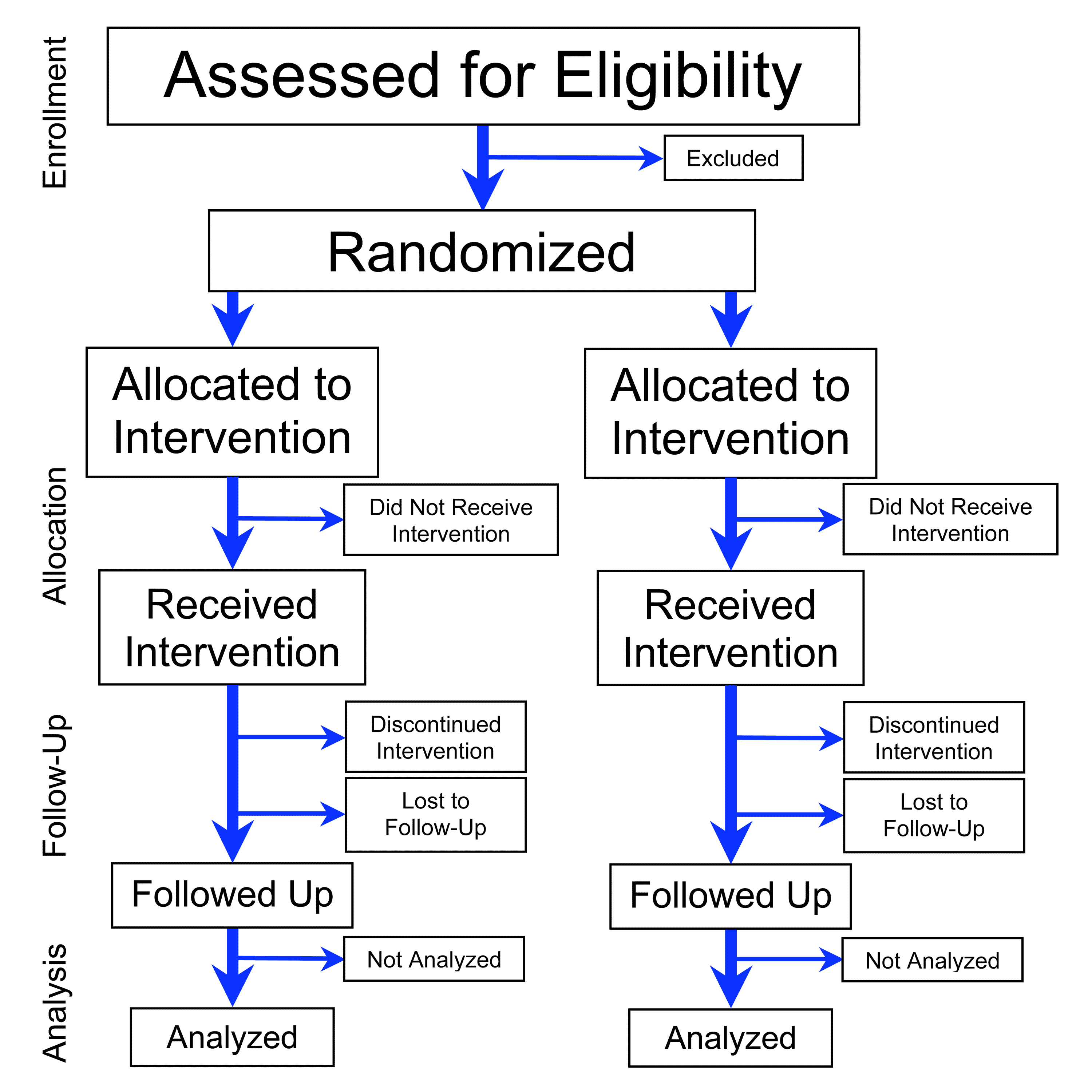
Randomized Controlled Trial
A randomized controlled trial (or randomized control trial; RCT) is a form of scientific experiment used to control factors not under direct experimental control. Examples of RCTs are clinical trials that compare the effects of drugs, surgical techniques, medical devices, diagnostic procedures, diets or other medical treatments. Participants who enroll in RCTs differ from one another in known and unknown ways that can influence study outcomes, and yet cannot be directly controlled. By randomly allocating participants among compared treatments, an RCT enables ''statistical control'' over these influences. Provided it is designed well, conducted properly, and enrolls enough participants, an RCT may achieve sufficient control over these confounding factors to deliver a useful comparison of the treatments studied. Definition and examples An RCT in clinical research typically compares a proposed new treatment against an existing standard of care; these are then termed the 'expe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Placebo-controlled Study
Placebo-controlled studies are a way of testing a medical therapy in which, in addition to a group of subjects that receives the treatment to be evaluated, a separate control group receives a sham "placebo" treatment which is specifically designed to have no real effect. Placebos are most commonly used in blinded trials, where subjects do not know whether they are receiving real or placebo treatment. Often, there is also a further "natural history" group that does not receive any treatment at all. The purpose of the placebo group is to account for the placebo effect, that is, effects from treatment that do not depend on the treatment itself. Such factors include knowing one is receiving a treatment, attention from health care professionals, and the expectations of a treatment's effectiveness by those running the research study. Without a placebo group to compare against, it is not possible to know whether the treatment itself had any effect. Patients frequently show improvement ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Design Of Experiments
The design of experiments (DOE), also known as experiment design or experimental design, is the design of any task that aims to describe and explain the variation of information under conditions that are hypothesized to reflect the variation. The term is generally associated with experiments in which the design introduces conditions that directly affect the variation, but may also refer to the design of quasi-experiments, in which natural conditions that influence the variation are selected for observation. In its simplest form, an experiment aims at predicting the outcome by introducing a change of the preconditions, which is represented by one or more independent variables, also referred to as "input variables" or "predictor variables." The change in one or more independent variables is generally hypothesized to result in a change in one or more dependent variables, also referred to as "output variables" or "response variables." The experimental design may also identify ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trials
Clinical trials are prospective biomedical or behavioral research studies on human subject research, human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, pharmaceutical drug, drugs, medical nutrition therapy, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received institutional review board, health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted. Depending on product type and development stage, investigators initially enroll volunteers or patients into small Pilot experiment, pi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scientific Method
The scientific method is an Empirical evidence, empirical method for acquiring knowledge that has been referred to while doing science since at least the 17th century. Historically, it was developed through the centuries from the ancient and medieval world. The scientific method involves careful observation coupled with rigorous skepticism, because Philosophy of science#Observation inseparable from theory, cognitive assumptions can distort the interpretation of the Perception#Process and terminology, observation. Scientific inquiry includes creating a testable hypothesis through inductive reasoning, testing it through experiments and statistical analysis, and adjusting or discarding the hypothesis based on the results. Although procedures vary across Branches of science, fields, the underlying #Process, process is often similar. In more detail: the scientific method involves making conjectures (hypothetical explanations), predicting the logical consequences of hypothesis, then ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |