|
Nylon
Nylon is a family of synthetic polymers characterised by amide linkages, typically connecting aliphatic or Polyamide#Classification, semi-aromatic groups. Nylons are generally brownish in color and can possess a soft texture, with some varieties exhibiting a silk-like appearance. As Thermoplastic, thermoplastics, nylons can be melt-processed into fibres, Thin film, films, and diverse shapes. The properties of nylons are often modified by blending with a variety of additives. Numerous types of nylon are available. One family, designated nylon-XY, is derived from diamines and dicarboxylic acids of carbon chain lengths X and Y, respectively. An important example is nylon-6,6 (). Another family, designated nylon-Z, is derived from amino acid, aminocarboxylic acids with carbon chain length Z. An example is nylon-[6]. Nylon polymers have extensive commercial applications, including uses in textiles and fibres (such as apparel, flooring and rubber reinforcement), molded components fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nylon 6 And Nylon 6-6
Nylon is a family of synthetic polymers characterised by amide linkages, typically connecting aliphatic or semi-aromatic groups. Nylons are generally brownish in color and can possess a soft texture, with some varieties exhibiting a silk-like appearance. As thermoplastics, nylons can be melt-processed into fibres, films, and diverse shapes. The properties of nylons are often modified by blending with a variety of additives. Numerous types of nylon are available. One family, designated nylon-XY, is derived from diamines and dicarboxylic acids of carbon chain lengths X and Y, respectively. An important example is nylon-6,6 (). Another family, designated nylon-Z, is derived from aminocarboxylic acids with carbon chain length Z. An example is nylon- Nylon polymers have extensive commercial applications, including uses in textiles and fibres (such as apparel, flooring and rubber reinforcement), molded components for automotive and electrical equipment, and films (mostly for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nylon 66
Nylon 66 (loosely written nylon 6-6, nylon 6/6, nylon 6,6, or nylon 6:6) is a type of polyamide or nylon. It, and nylon 6, are the two most common for textile and plastic industries. Nylon 66 is made of two monomers each containing six carbon atoms, hexamethylenediamine and adipic acid, which give nylon 66 its name. Aside from its superior physical characteristics, nylon 66 is attractive because its precursors are inexpensive. Synthesis and manufacturing Hexamethylenediamine (top) and adipic acid (bottom), monomers used for polycondensation of Nylon 66. Nylon 66 is synthesized by polycondensation of hexamethylenediamine and adipic acid. Equivalent amounts of hexamethylenediamine and adipic acid are combined in water. In the original implementation, the resulting ammonium/ carboxylate salt was isolated and then heated either in batches or continuously to induce polycondensation. : (2n-1) Removing water drives the reaction toward polymerization through the formation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyamide
A polyamide is a polymer with repeating units linked by amide bonds. Polyamides occur both naturally and artificially. Examples of naturally occurring polyamides are proteins, such as wool and silk. Artificially made polyamides can be made through step-growth polymerization or solid-phase synthesis yielding materials such as nylons, aramids, and sodium polyaspartate. Synthetic polyamides are commonly used in textiles, automotive industry, carpets, kitchen utensils and sportswear due to their high durability and strength. The transportation manufacturing industry is the major consumer, accounting for 35% of polyamide (PA) consumption. Classification Polymers of amino acids are known as polypeptides or proteins. According to the composition of their main chain, synthetic polyamides are classified as follows: All polyamides are made by the formation of an amide function to link two molecules of monomer together. The monomers can be amides themselves (usually in the form of a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Textiles
Textile is an Hyponymy and hypernymy, umbrella term that includes various Fiber, fiber-based materials, including fibers, yarns, Staple (textiles)#Filament fiber, filaments, Thread (yarn), threads, and different types of #Fabric, fabric. At first, the word "textiles" only referred to woven fabrics. However, weaving is not the only manufacturing method, and many other methods were later developed to form textile structures based on their intended use. Knitting and Nonwoven, non-woven are other popular types of fabric manufacturing. In the contemporary world, textiles satisfy the material needs for versatile applications, from simple daily clothing to Bulletproof vest, bulletproof jackets, spacesuits, and Medical gown, doctor's gowns. Textiles are divided into two groups: consumer textiles for domestic purposes and technical textiles. In consumer textiles, Aesthetics (textile), aesthetics and Textile performance#Comfort, comfort are the most important factors, while in techn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wallace Hume Carothers
Wallace Hume Carothers (; April 27, 1896 – April 29, 1937) was an American chemist, inventor, and the leader of organic chemistry at DuPont, who was credited with the invention of nylon. Carothers was a group leader at the DuPont Experimental Station laboratory, near Wilmington, Delaware, where most polymer research was done. Carothers was an organic chemist who, in addition to first developing nylon, also helped lay the groundwork for neoprene. After receiving his Ph.D., he taught at several universities before he was hired by DuPont to work on fundamental research. He married Helen Sweetman on February 21, 1936. Carothers had been troubled by periods of depression since his youth. Despite his success with nylon, he felt that he had not accomplished much and had run out of ideas. His unhappiness was exacerbated by the death of his sister, and on April 28, 1937, he committed suicide by drinking potassium cyanide. His daughter, Jane, was born on November 27, 1937. Educa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Polymer
Some familiar household synthetic polymers include: Nylons in textiles and fabrics, Teflon in non-stick pans, Bakelite for electrical switches, polyvinyl chloride (PVC) in pipes, etc. The common PET bottles are made of a synthetic polymer, polyethylene terephthalate. The plastic kits and covers are mostly made of synthetic polymers like polythene, and tires are manufactured from polybutadienes. However, due to the environmental issues created by these synthetic polymers which are mostly non-biodegradable and often synthesized from petroleum, alternatives like bioplastics are also being considered. They are however expensive when compared to the synthetic polymers. Inorganic polymers * Polysiloxane * Polyphosphazene * Polyborazyline Organic polymers The eight most common types of synthetic organic polymers, which are commonly found in households are: * Low-density polyethylene (LDPE) *High-density polyethylene (HDPE) *Polypropylene (PP) *Polyethylene (PE) *Polyvinyl chl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Charles Stine
Charles Milton Altland Stine (18 October 1882 – 28 May 1954) was a chemist and a vice-president of DuPont who created the laboratory from which nylon and other significant inventions were made. He was also a devout Christian who authored a book about religion and science. Stine was born in Norwich, Connecticut to Lutheran clergyman Milton Henry Stine and his wife Mary Jane Altland. He received his BS and MS degrees from Gettysburg college and then received a Ph.D. from Johns Hopkins University in 1907, Stine began work in DuPont's research laboratories on a project to make explosives safer to handle. With C.C. Ahlum, he used sodium sulfite as a purifying agent to crystallize trinitrotoluol ( TNT). After studying the leakage of liquid components from dynamite, Stine was able to develop a more stable version of the explosive for use in mining. He developed improved methods for manufacture of ammonium nitrate, extraction of tetryl from dimethylaniline, picric acid from chlor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silk
Silk is a natural fiber, natural protein fiber, some forms of which can be weaving, woven into textiles. The protein fiber of silk is composed mainly of fibroin and is most commonly produced by certain insect larvae to form cocoon (silk), cocoons. The best-known silk is obtained from the cocoons of the larvae of the mulberry silkworm ''Bombyx mori'' reared in captivity (sericulture). The shimmering appearance of silk is due to the triangular Prism (optics), prism-like structure of the silk fibre, which allows silk cloth to refract incoming light at different angles, thus producing different colors. Harvested silk is produced by several insects; but, generally, only the silk of various moth caterpillars has been used for textile manufacturing. There has been some research into other types of silk, which differ at the molecular level. Silk is mainly produced by the larvae of insects undergoing holometabolism, complete metamorphosis, but some insects, such as webspinners and Gr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the Polymer backbone, main chain of a protein, and an isopeptide bond when it occurs in a side chain, as in asparagine and glutamine. It can be viewed as a Derivative (chemistry), derivative of a carboxylic acid () with the hydroxyl group () replaced by an amino group (); or, equivalently, an acyl group, acyl (alkanoyl) group () joined to an amino group. Common amides are formamide (), acetamide (), benzamide (), and dimethylformamide (). Some uncommon examples of amides are ''N''-chloroacetamide () and chloroformamide (). Amides are qualified as primary (chemistry), primary, secondary (chemistry), secondary, and tertiary (chemistry), tertiary according to the number of acyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
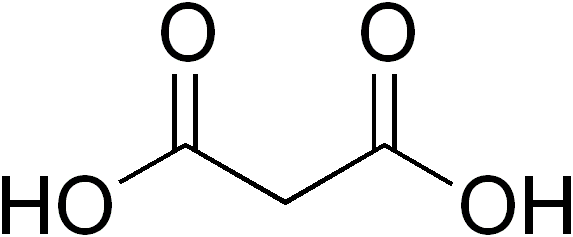
Dicarboxylic Acid
In organic chemistry, a dicarboxylic acid is an organic compound containing two carboxyl groups (). The general molecular formula for dicarboxylic acids can be written as , where R can be aliphatic or aromatic.Boy Cornils, Peter Lappe "Dicarboxylic Acids, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry 2014, Wiley-VCH, Weinheim. In general, dicarboxylic acids show similar chemical behavior and reactivity to monocarboxylic acids. Dicarboxylic acids are usually colorless solids. A wide variety of dicarboxylic acids are used in industry. Adipic acid, for example, is a precursor to certain kinds of nylon. A wide variety of dicarboxylic acids are found in nature. Aspartic acid and glutamic acid are two amino acids found in all life. Succinic and fumaric acids are essential for metabolism. A large inventory of derivatives are known including many mono- and diesters, amides, etc. Partial list of saturated dicarboxylic acids Some common or illustrative examples : ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Science History Institute
The Science History Institute is an institution that preserves and promotes understanding of the history of science. Located in Philadelphia, Pennsylvania, it includes a library, museum, archive, research center and conference center. It was founded in 1982 as a joint venture of the American Chemical Society and the University of Pennsylvania, as the Center for the History of Chemistry (CHOC). The American Institute of Chemical Engineers (AIChE) became a co-founder in 1984. It was renamed the Chemical Heritage Foundation (CHF) in 1992, and moved two years later to the institution's current location, 315 Chestnut Street in Old City. On December 1, 2015, CHF merged with the Life Sciences Foundation, creating an organization that covers "the history of the life sciences and biotechnology together with the history of the chemical sciences and engineering." As of February 1, 2018, the organization was renamed the Science History Institute, to reflect its wider range of histori ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermoplastic
A thermoplastic, or thermosoftening plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling. Most thermoplastics have a high molecular weight. The polymer chains associate by intermolecular forces, which weaken rapidly with increased temperature, yielding a viscous liquid. In this state, thermoplastics may be reshaped, and are typically used to produce parts by various polymer processing techniques such as injection molding, compression molding, calendering, and extrusion. Thermoplastics differ from thermosetting polymers (or "thermosets"), which form irreversible chemical bonds during the curing process. Thermosets do not melt when heated, but typically decompose and do not reform upon cooling. Above its glass transition temperature and below its melting point, the physical properties of a thermoplastic change drastically without an associated phase change. Some thermoplastics do not fully ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |