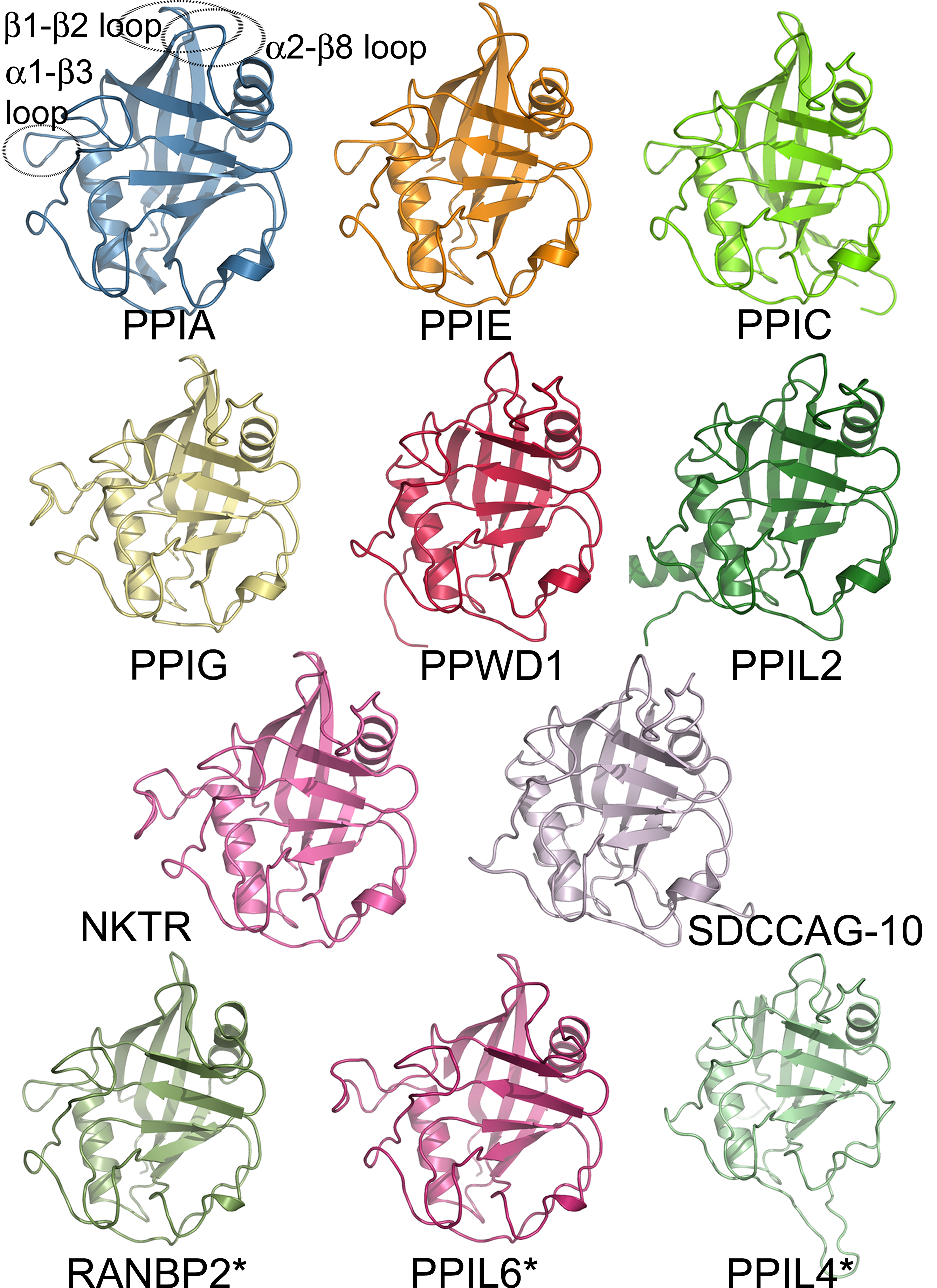
|
Nuclear Pore
The nuclear pore complex (NPC), is a large protein complex giving rise to the nuclear pore. A great number of nuclear pores are studded throughout the nuclear envelope that surrounds the eukaryote cell nucleus. The pores enable the nuclear transport of macromolecules between the nucleoplasm of the nucleus and the cytoplasm of the Cell (biology), cell. Small molecules can easily Passive diffusion, diffuse through the pores. Nuclear transport includes the transportation of RNA and ribosomal proteins from the nucleus to the cytoplasm, and the transport of proteins (such as DNA polymerase and lamins), carbohydrates, signaling molecules, and lipids into the nucleus. Each nuclear pore complex can actively mediate up to 1000 translocations per second. The nuclear pore complex consists predominantly of a Protein family, family of proteins known as nucleoporins (Nups). Each pore complex in the human cell nucleus is composed of about 1,000 individual protein molecules, from an Conserved s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Complex
A protein complex or multiprotein complex is a group of two or more associated polypeptide chains. Protein complexes are distinct from multidomain enzymes, in which multiple active site, catalytic domains are found in a single polypeptide chain. Protein complexes are a form of protein quaternary structure, quaternary structure. Proteins in a protein complex are linked by non-covalent interactions, non-covalent protein–protein interactions. These complexes are a cornerstone of many (if not most) biological processes. The cell is seen to be composed of modular supramolecular complexes, each of which performs an independent, discrete biological function. Through proximity, the speed and selectivity of binding interactions between Enzyme, enzymatic complex and substrates can be vastly improved, leading to higher cellular efficiency. Many of the techniques used to enter cells and isolate proteins are inherently disruptive to such large complexes, complicating the task of determining ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Family
A protein family is a group of evolutionarily related proteins. In many cases, a protein family has a corresponding gene family, in which each gene encodes a corresponding protein with a 1:1 relationship. The term "protein family" should not be confused with family as it is used in taxonomy. Proteins in a family descend from a common ancestor and typically have similar three-dimensional structures, functions, and significant sequence similarity. Sequence similarity (usually amino-acid sequence) is one of the most common indicators of homology, or common evolutionary ancestry. Some frameworks for evaluating the significance of similarity between sequences use sequence alignment methods. Proteins that do not share a common ancestor are unlikely to show statistically significant sequence similarity, making sequence alignment a powerful tool for identifying the members of protein families. Families are sometimes grouped together into larger clades called superfamilies based on st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylalanine
Phenylalanine (symbol Phe or F) is an essential α-amino acid with the chemical formula, formula . It can be viewed as a benzyl group substituent, substituted for the methyl group of alanine, or a phenyl group in place of a terminal hydrogen of alanine. This essential amino acid is classified as neutral, and chemical polarity, nonpolar because of the inert and hydrophobic nature of the benzyl side chain. The chirality (chemistry)#Naming conventions, L-isomer is used to biochemically form proteins coded for by DNA. Phenylalanine is a precursor for tyrosine, the monoamine neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), and the biological pigment melanin. It is Genetic code, encoded by the messenger RNA codons UUU and UUC. Phenylalanine is found naturally in the milk of mammals. It is used in the manufacture of food and drink products and sold as a nutritional supplement as it is a direct precursor to the neuromodulation, neuromodulator phe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FG Nucleoporin
Nucleoporins are a family of proteins which are the constituent building blocks of the nuclear pore complex (NPC). The nuclear pore complex is a massive structure embedded in the nuclear envelope at sites where the inner and outer nuclear membranes fuse, forming a gateway that regulates the flow of macromolecules between the cell nucleus and the cytoplasm. Nuclear pores enable the passive and facilitated transport of molecules across the nuclear envelope. Nucleoporins, a family of around 30 proteins, are the main components of the nuclear pore complex in eukaryotic cells. Nucleoporin 62 is the most abundant member of this family. Nucleoporins are able to transport molecules across the nuclear envelope at a very high rate. A single NPC is able to transport 60,000 protein molecules across the nuclear envelope every minute. Structure Nucleoporins aggregate to form a nuclear pore complex, an octagonal ring that traverses the nuclear envelope. The ring consists of eight sca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tertiary Structure
Protein tertiary structure is the three-dimensional shape of a protein. The tertiary structure will have a single polypeptide chain "backbone" with one or more protein secondary structures, the protein domains. Amino acid side chains and the backbone may interact and bond in a number of ways. The interactions and bonds of side chains within a particular protein determine its tertiary structure. The protein tertiary structure is defined by its atomic coordinates. These coordinates may refer either to a protein domain or to the entire tertiary structure. A number of these structures may bind to each other, forming a quaternary structure. History The science of the tertiary structure of proteins has progressed from one of hypothesis to one of detailed definition. Although Emil Fischer had suggested proteins were made of polypeptide chains and amino acid side chains, it was Dorothy Maud Wrinch who incorporated geometry into the prediction of protein structures. Wrinch demon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intrinsically Disordered Proteins
In molecular biology, an intrinsically disordered protein (IDP) is a protein that lacks a fixed or ordered protein tertiary structure, three-dimensional structure, typically in the absence of its macromolecular interaction partners, such as other proteins or RNA. IDPs range from fully unstructured to partially structured and include random coil, molten globule-like Protein aggregation, aggregates, or flexible linkers in large multi-Protein domain, domain proteins. They are sometimes considered as a separate class of proteins along with globular protein, globular, fibrous protein, fibrous and membrane proteins. IDPs are a very large and functionally important class of proteins. They are most numerous in Eukaryote, eukaryotes, with an estimated 30-40% of residues in the eukaryotic proteome located in disordered regions. Disorder is present in around 70% of proteins, either in the form of disordered tails or flexible linkers. Proteins can also be entirely disordered and lack a define ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structural Domain
In molecular biology, a protein domain is a region of a protein's Peptide, polypeptide chain that is self-stabilizing and that Protein folding, folds independently from the rest. Each domain forms a compact folded Protein tertiary structure, three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or Disulfide bond, disulfide bridges. Domains often form functional units, such as the calcium-binding EF-hand, EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimera (protein), chimeric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta-propeller
In structural biology, a beta-propeller (β-propeller) is a type of all-β protein architecture characterized by 4 to 8 highly symmetrical blade-shaped beta sheets arranged toroidally around a central axis. Together the beta-sheets form a funnel-like active site. Structure Each beta-sheet typically has four anti-parallel β-strands arranged in the beta-zigzag motif. The strands are twisted so that the first and fourth strands are almost perpendicular to each other. There are five classes of beta-propellers, each arrangement being a highly symmetrical structure with 4–8 beta sheets, all of which generally form a central tunnel that yields pseudo-symmetric axes. While, the protein's official active site for ligand-binding is formed at one end of the central tunnel by loops between individual beta-strands, protein-protein interactions can occur at multiple areas around the domain. Depending on the packing and tilt of the beta-sheets and beta-strands, the beta-propeller may hav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Solenoid
An alpha solenoid (sometimes also known as an alpha horseshoe or as stacked pairs of alpha helices, abbreviated SPAH) is a protein fold composed of repeating alpha helix subunits, commonly helix-turn-helix motifs, arranged in antiparallel fashion to form a superhelix. Alpha solenoids are known for their flexibility and plasticity. Like beta propellers, alpha solenoids are a form of solenoid protein domain commonly found in the proteins comprising the nuclear pore complex. They are also common in membrane coat proteins known as coatomers, such as clathrin, and in regulatory proteins that form extensive protein-protein interactions with their binding partners. Examples of alpha solenoid structures binding RNA and lipids have also been described. Terminology and classification The term "alpha solenoid" has been used somewhat inconsistently in the literature. As originally defined, alpha solenoids were composed of helix-turn-helix motifs that stacked into an open superhelix. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solenoid Protein Domain
Solenoid protein domains are a highly modular type of protein domain. They consist of a chain of nearly identical Protein structure, folds, often simply called Protein tandem repeats, tandem repeats. They are extremely common among all types of proteins, though exact figures are unknown. "Repeats" in molecular biology In proteins, a "repeat" is any sequence block that returns more than one time in the protein sequence, sequence, either in an identical or a highly similar form. Repetitiveness does not in itself indicate anything about the structure of the protein. As a "rule of thumb", short repetitive sequences (e.g. those below the length of 10 amino acids) may be intrinsically unstructured proteins, intrinsically disordered, and not part of any protein folding, folded protein domains. Repeats that are at least 30 to 40 amino acids long, are far more likely to be folded as part of a domain. Such long repeats are frequently indicative of the presence of a solenoid domain in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Basket
The cell nucleus (; : nuclei) is a membrane-bound organelle found in eukaryotic cells. Eukaryotic cells usually have a single nucleus, but a few cell types, such as mammalian red blood cells, have no nuclei, and a few others including osteoclasts have many. The main structures making up the nucleus are the nuclear envelope, a double membrane that encloses the entire organelle and isolates its contents from the cellular cytoplasm; and the nuclear matrix, a network within the nucleus that adds mechanical support. The cell nucleus contains nearly all of the cell's genome. Nuclear DNA is often organized into multiple chromosomes – long strands of DNA dotted with various proteins, such as histones, that protect and organize the DNA. The genes within these chromosomes are structured in such a way to promote cell function. The nucleus maintains the integrity of genes and controls the activities of the cell by regulating gene expression. Because the nuclear envelope is impermeabl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ran Cycle
Ran (RAs-related Nuclear protein) also known as GTP-binding nuclear protein Ran is a protein that in humans is encoded by the RAN gene. Ran is a small 25 kDa protein that is involved in transport into and out of the cell nucleus during interphase and also involved in mitosis. It is a member of the Ras superfamily. Ran is a small G protein that is essential for the translocation of RNA and proteins through the nuclear pore complex. The Ran protein has also been implicated in the control of DNA synthesis and cell cycle progression, as mutations in Ran have been found to disrupt DNA synthesis. Function Ran cycle Ran exists in the cell in two nucleotide-bound forms: GDP-bound and GTP-bound. RanGDP is converted into RanGTP through the action of RCC1, the nucleotide exchange factor for Ran. RCC1 is also known as RanGEF (Ran Guanine nucleotide Exchange Factor). Ran's intrinsic GTPase-activity is activated through interaction with Ran GTPase activating protein (RanGAP), facili ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |