|
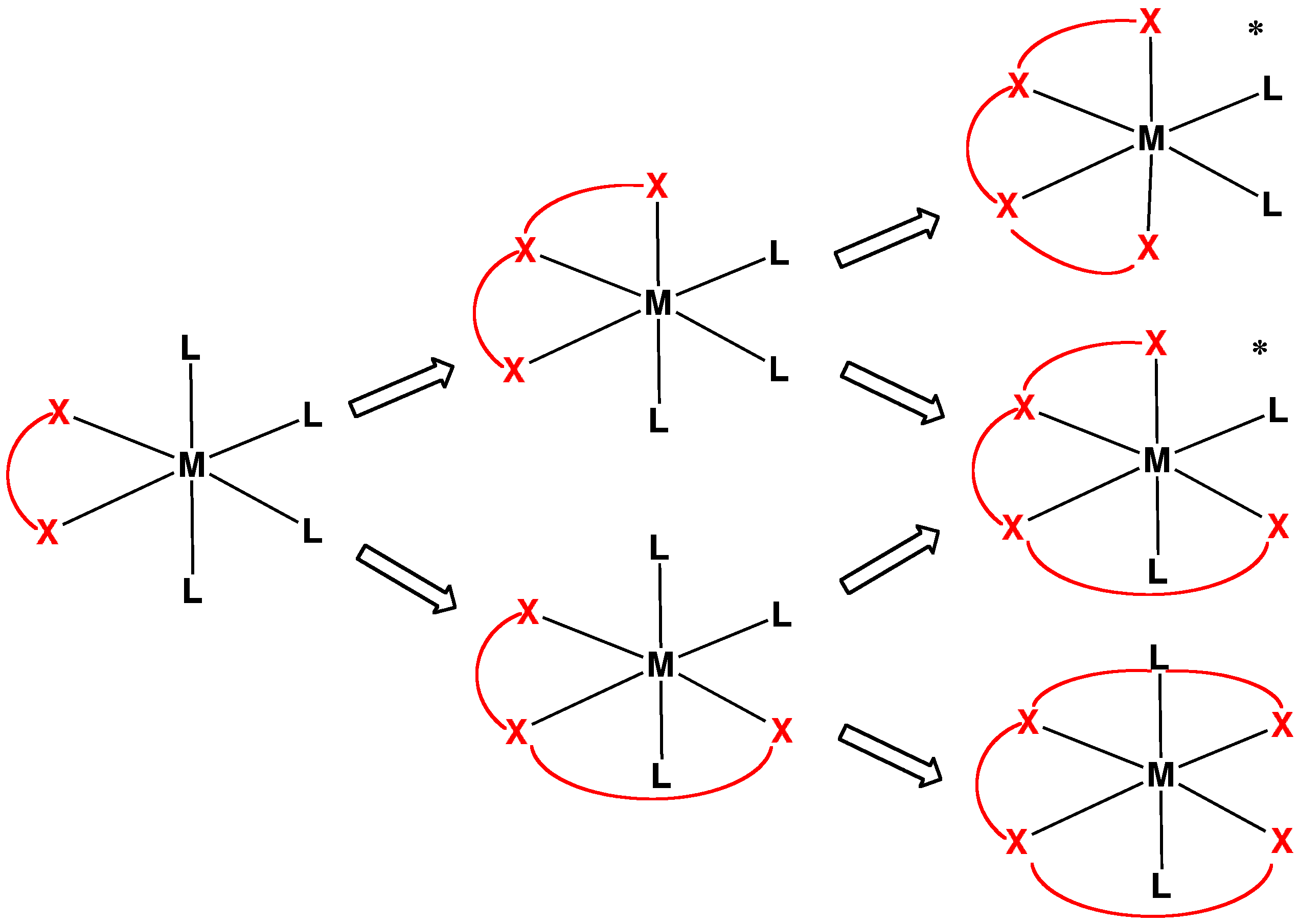
Monodentate
In coordination chemistry, denticity () refers to the number of donor groups in a given ligand that bind to the central metal atom in a coordination complex. In many cases, only one atom in the ligand binds to the metal, so the denticity equals one, and the ligand is said to be monodentate (sometimes called unidentate). Ligands with more than one bonded atom are called polydentate or multidentate. The denticity of a ligand is described with the Greek letter κ ('kappa'). For example, κ6-EDTA describes an EDTA ligand that coordinates through 6 non-contiguous atoms. Denticity is different from hapticity because hapticity refers exclusively to ligands where the coordinating atoms are contiguous. In these cases the η ('eta') notation is used. Bridging ligands use the μ ('mu') notation. Classes Polydentate ligands are chelating agents and classified by their denticity. Some atoms cannot form the maximum possible number of bonds a ligand could make. In that case one or mor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environment ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chelating Agents
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity, as in the case of zinc and its use as a maintenance therapy to prevent the absorption of copper in people with Wilson's disease. Chelation is useful in applications such as providing nutritional supplements, in chelation therapy to remove toxic metals from the body, as contrast agents in MRI scanning, in manufacturing using homogeneous catalysts, in chemical water treatment to assist in the removal of metals, and in fertilizers. Chelate effect The chelate effect is the greater affinity of chelating ligands for a metal ion than that of similar nonchelating (monodentate) ligands for the same met ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Chemistry
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing compounds, especially those that include transition metals (elements like titanium that belong to the Periodic Table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom through several of the ligand's atoms; ligands with 2, 3, 4 or even 6 bonds to the central atom are common. These com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as '' ligands'' or complexing agents. Many metal-containing compounds, especially those that include transition metals (elements like titanium that belong to the Periodic Table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom through several of the ligand's atoms; ligands with 2, 3, 4 or even 6 bonds to the central atom are common. These ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetradentate Ligand
In chemistry, tetradentate ligands are ligands that bind four donor atoms to a central atom to form a coordination complex. This number of donor atoms that bind is called denticity and is a method of classifying ligands. Tetradentate ligands are common in nature in the form of chlorophyll, which has a core ligand called chlorin, and heme, which has a core ligand called porphyrin. They are responsible for the colour observed in plants and humans. Phthalocyanine is an artificial macrocyclic tetradentate ligand that is used to make blue and green pigments. Shape Tetradentate ligands can be classified by the topology of the connections between donor atoms. Common forms are linear (also called sequential), ring or tripodal. A tetrapodal ligand that is also tetradentate has four legs with donor atoms and a bridgehead that is not a donor. Upon binding with a central atom, there are several arrangements possible (known as geometric isomers). Linear ligands A linear tetradentate lig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxaliplatin
Oxaliplatin, sold under the brand name Eloxatin among others, is a cancer medication (platinum-based antineoplastic class) used to treat colorectal cancer. It is given by injection into a vein. Common side effects include numbness, feeling tired, nausea, diarrhea, and low blood cell counts. Other serious side effects include allergic reactions. Use in pregnancy is known to harm the baby. Oxaliplatin is in the platinum-based antineoplastic family of medications. It is believed to work by blocking the duplication of DNA. Oxaliplatin was patented in 1976 and approved for medical use in 1996. It is on the World Health Organization's List of Essential Medicines. Medical uses Oxaliplatin is used for treatment of colorectal cancer, typically along with folinic acid (leucovorin) and fluorouracil in a combination known as FOLFOX or along with capecitabine in a combination known as CAPOX or XELOX. Advanced colorectal cancer Oxaliplatin by itself has modest activity ag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triethylenetetramine
Triethylenetetramine (TETA and trien), also known as trientine (INN) when used medically, is an organic compound with the formula H2NHCH2CH2NH2sub>2. The pure freebase is a colorless oily liquid, but, like many amines, older samples assume a yellowish color due to impurities resulting from air-oxidation. It is soluble in polar solvents. The branched isomer tris(2-aminoethyl)amine and piperazine derivatives may also be present in commercial samples of TETA. The hydrochloride salts are used medically as a treatment for copper toxicity. Uses The reactivity and uses of TETA are similar to those for the related polyamines ethylenediamine and diethylenetriamine. It is primarily used as a crosslinker ("hardener") in epoxy curing. Medical uses The hydrochloride salt of TETA, referred to as ''trientine hydrochloride'', is a chelating agent that is used to bind and remove copper in the body to treat Wilson's disease, particularly in those who are intolerant to penicillamine. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tris(2-aminoethyl)amine
Tris(2-aminoethyl)amine is the organic compound with the formula N(CH2CH2NH2)3. This colourless liquid is soluble in water and is highly basic, consisting of a tertiary amine center and three pendant primary amine groups. Abbreviated tren or TREN it is a crosslinking agent in the synthesis of polyimine networks and a tripodal ligand in coordination chemistry. Tren is a C3-symmetric, tetradentate chelating ligand that forms stable complexes with transition metals, especially those in the 2+ and 3+ oxidation states. Tren complexes exist with relatively few isomers, reflecting the constrained connectivity of this tetramine. Thus, only a single achiral stereoisomer exists for o(tren)X2sup>+, where X is halide or pseudohalide. In contrast, for o(trien)X2sup>+ five diastereomers are possible, four of which are chiral. In a few cases, tren serves as a tridentate ligand with one of the primary amine groups non-coordinated. Tren is a common impurity in the more common triethylenetetr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Macrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry. Synthesis The formation of macrocycles by ring-closure is called macrocylization. Pioneering work was reported for studies on terpenoid macrocycles. The central challenge to macrocyclization is that ring-closing reactions do not favor the formation of large rings. Instead, small rings or polymers tend to form. This kinetic problem can be addressed by using high-dilution reactions, whereby intramolecular processes are favored relative to polymerizations. Some macrocyclizations are favored using template reactions. Templates are ions, molecules, surfaces etc. that bind and pre-organize compounds, guiding them toward formation of a particular ring size. The crown ethers are often generated in the presence of an alkali metal cation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Porphyrin
Porphyrins ( ) are a group of heterocyclic macrocycle organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (=CH−). The parent of porphyrin is porphine, a rare chemical compound of exclusively theoretical interest. Substituted porphines are called porphyrins. With a total of 26 π-electrons, of which 18 π-electrons form a planar, continuous cycle, the porphyrin ring structure is often described as aromatic. One result of the large conjugated system is that porphyrins typically absorb strongly in the visible region of the electromagnetic spectrum, i.e. they are deeply colored. The name "porphyrin" derives from the Greek word πορφύρα (''porphyra''), meaning ''purple''. Complexes of porphyrins Concomitant with the displacement of two N-''H'' protons, porphyrins bind metal ions in the N4 "pocket". The metal ion usually has a charge of 2+ or 3+. A schematic equation for these syntheses is shown: :H2porp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,4,7-Trithiacyclononane
1,4,7-Trithiacyclononane, also called 9-ane-S3, is the thia-crown ether with the formula (CH2CH2S)3. This cyclic thioether is most often encountered as a tridentate ligand in coordination chemistry, where it forms transition metal thioether complexes. 9-ane-S3 forms complexes with many metal ions, including those considered hard, such as copper(II) and iron(II). Most of its complexes have the formula (9-ane-S3)2sup>2+ and are octahedral. The point group of (9-ane-S3)2sup>2+ is S6. Synthesis This compound was first reported in 1977, and the current synthesis entails the assembly within the coordination sphere In coordination chemistry, the first coordination sphere refers to the array of molecules and ions (the ligands) directly attached to the central metal atom. The second coordination sphere consists of molecules and ions that attached in various ... of a metal ion followed by decomplexation: : References {{DEFAULTSORT:Trithiacyclononane, 1,4,7- Chelating agent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

4-3D-balls.png)