|
Methylxanthine
Xanthine ( or ; archaically xanthic acid; systematic name 3,7-dihydropurine-2,6-dione) is a purine base found in most human body tissues and fluids, as well as in other organisms. Several stimulants are derived from xanthine, including caffeine, theophylline, and theobromine. Xanthine is a product on the pathway of purine degradation. * It is created from guanine by guanine deaminase. * It is created from hypoxanthine by xanthine oxidoreductase. * It is also created from xanthosine by purine nucleoside phosphorylase. Xanthine is subsequently converted to uric acid by the action of the xanthine oxidase enzyme. Use and manufacturing Xanthine is used as a drug precursor for human and animal medications, and is manufactured as a pesticide ingredient. Clinical significance Derivatives of xanthine (known collectively as xanthines) are a group of alkaloids commonly used for their effects as mild stimulants and as bronchodilators, notably in the treatment of asthma or influenza sympto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caffeine
Caffeine is a central nervous system (CNS) stimulant of the methylxanthine class. It is mainly used recreationally as a cognitive enhancer, increasing alertness and attentional performance. Caffeine acts by blocking binding of adenosine to the adenosine A1 receptor, which enhances release of the neurotransmitter acetylcholine. Caffeine has a three-dimensional structure similar to that of adenosine, which allows it to bind and block its receptors. Caffeine also increases cyclic AMP levels through nonselective inhibition of phosphodiesterase. Caffeine is a bitter, white crystalline purine, a methylxanthine alkaloid, and is chemically related to the adenine and guanine bases of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). It is found in the seeds, fruits, nuts, or leaves of a number of plants native to Africa, East Asia and South America, and helps to protect them against herbivores and from competition by preventing the germination of nearby seeds, as well as en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine
Adenosine (symbol A) is an organic compound that occurs widely in nature in the form of diverse derivatives. The molecule consists of an adenine attached to a ribose via a β-N9- glycosidic bond. Adenosine is one of the four nucleoside building blocks of RNA (and its derivative deoxyadenosine is a building block of DNA), which are essential for all life. Its derivatives include the energy carriers adenosine mono-, di-, and triphosphate, also known as AMP/ADP/ATP. Cyclic adenosine monophosphate (cAMP) is pervasive in signal transduction. Adenosine is used as an intravenous medication for some cardiac arrhythmias. Adenosyl (abbreviated Ado or 5'-dAdo) is the chemical group formed by removal of the 5′-hydroxy (OH) group. It is found in adenosylcobalamin (an active form of vitamin B12) and as a radical in radical SAM enzymes. Medical uses Supraventricular tachycardia In individuals with supraventricular tachycardia (SVT), adenosine is used to help identify and convert t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paraxanthine
Paraxanthine, or 1,7-dimethylxanthine, is a dimethyl derivative of xanthine, structurally related to caffeine. Production and metabolism Paraxanthine is not known to be produced by plants but is observed in nature as a metabolite of caffeine in animals and some species of bacteria. After intake, roughly 84% of caffeine is demethylated at the 3-position to yield paraxanthine, making it the primary metabolite of caffeine in humans. Paraxanthine is also a major metabolite of caffeine in humans and other animals, such as mice. Shortly after ingestion, caffeine is metabolized into paraxanthine by hepatic cytochrome P450, which removes a methyl group from the N3 position of caffeine. After formation, paraxanthine can be broken down to 7-methylxanthine by demethylation of the N1 position, which is subsequently demethylated into xanthine or oxidized by CYP2A6 and CYP1A2 into 1,7-dimethyluric acid. In another pathway, paraxanthine is broken down into 5-acetylamino-6-formylamino-3-methylu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Asthma
Asthma is a long-term inflammatory disease of the airways of the lungs. It is characterized by variable and recurring symptoms, reversible airflow obstruction, and easily triggered bronchospasms. Symptoms include episodes of wheezing, coughing, chest tightness, and shortness of breath. These may occur a few times a day or a few times per week. Depending on the person, asthma symptoms may become worse at night or with exercise. Asthma is thought to be caused by a combination of genetic and environmental factors. Environmental factors include exposure to air pollution and allergens. Other potential triggers include medications such as aspirin and beta blockers. Diagnosis is usually based on the pattern of symptoms, response to therapy over time, and spirometry lung function testing. Asthma is classified according to the frequency of symptoms, forced expiratory volume in one second (FEV1), and peak expiratory flow rate. It may also be classified as atopic or non-atop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Theophylline
Theophylline, also known as 1,3-dimethylxanthine, is a phosphodiesterase inhibiting drug used in therapy for respiratory diseases such as chronic obstructive pulmonary disease (COPD) and asthma under a variety of brand names. As a member of the xanthine family, it bears structural and pharmacological similarity to theobromine and caffeine, and is readily found in nature, being present in tea (''Camellia sinensis'') and cocoa (''Theobroma cacao''). A small amount of theophylline is one of the products of caffeine metabolic processing in the liver. Medical uses The main actions of theophylline involve: * relaxing bronchial smooth muscle * increasing heart muscle contractility and efficiency (positive inotrope) * increasing heart rate (positive chronotropic) * increasing blood pressure * increasing renal blood flow * anti-inflammatory effects * central nervous system stimulatory effect mainly on the medullary respiratory center. The main therapeutic uses of theophylline are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Theobromine
Theobromine, also known as xantheose, is the principal alkaloid of '' Theobroma cacao'' (cacao plant). Theobromine is slightly water-soluble (330 mg/L) with a bitter taste. In industry, theobromine is used as an additive and precursor to some cosmetics. It is found in chocolate, as well as in a number of other foods, including the leaves of the tea plant, and the kola nut. It is a white or colourless solid, but commercial samples can appear yellowish. Structure Theobromine is a flat molecule, a derivative of purine. It is also classified as a di methyl xanthine. Related compounds include theophylline, caffeine, paraxanthine, and 7-methylxanthine, each of which differ in the number or placement of the methyl groups. History Theobromine was first discovered in 1841 in cacao beans by Russian chemist Aleksandr Voskresensky. Synthesis of theobromine from xanthine was first reported in 1882 by Hermann Emil Fischer. Etymology ''Theobromine'' is derived from '' Theobroma'', ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
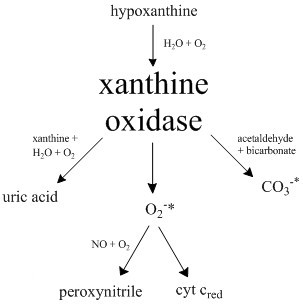
Xanthine Oxidase
Xanthine oxidase (XO, sometimes XAO) is a form of xanthine oxidoreductase, a type of enzyme that generates reactive oxygen species. These enzymes catalyze the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid. These enzymes play an important role in the catabolism of purines in some species, including humans. Xanthine oxidase is defined as an ''enzyme activity'' (EC 1.17.3.2). The same protein, which in humans has the HGNC approved gene symbol ''XDH'', can also have xanthine dehydrogenase activity (EC 1.17.1.4). Most of the protein in the liver exists in a form with xanthine dehydrogenase activity, but it can be converted to xanthine oxidase by reversible sulfhydryl oxidation or by irreversible proteolytic modification. Reaction The following chemical reactions are catalyzed by xanthine oxidase: * hypoxanthine + H2O + O2 \rightleftharpoons xanthine + H2O2 * xanthine + H2O + O2 \rightleftharpoons uric acid + H2O2 * Xanthine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xanthine Oxidoreductase
Xanthine oxidase (XO, sometimes XAO) is a form of xanthine oxidoreductase, a type of enzyme that generates reactive oxygen species. These enzymes catalyze the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid. These enzymes play an important role in the catabolism of purines in some species, including humans. Xanthine oxidase is defined as an ''enzyme activity'' (EC 1.17.3.2). The same protein, which in humans has the HGNC approved gene symbol ''XDH'', can also have xanthine dehydrogenase activity (EC 1.17.1.4). Most of the protein in the liver exists in a form with xanthine dehydrogenase activity, but it can be converted to xanthine oxidase by reversible sulfhydryl oxidation or by irreversible proteolytic modification. Reaction The following chemical reactions are catalyzed by xanthine oxidase: * hypoxanthine + H2O + O2 \rightleftharpoons xanthine + H2O2 * xanthine + H2O + O2 \rightleftharpoons uric acid + H2O2 * Xanthine o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Influenza
Influenza, commonly known as "the flu", is an infectious disease caused by influenza viruses. Symptoms range from mild to severe and often include fever, runny nose, sore throat, muscle pain, headache, coughing, and fatigue. These symptoms begin from one to four days after exposure to the virus (typically two days) and last for about 2–8 days. Diarrhea and vomiting can occur, particularly in children. Influenza may progress to pneumonia, which can be caused by the virus or by a subsequent bacterial infection. Other complications of infection include acute respiratory distress syndrome, meningitis, encephalitis, and worsening of pre-existing health problems such as asthma and cardiovascular disease. There are four types of influenza virus, termed influenza viruses A, B, C, and D. Aquatic birds are the primary source of Influenza A virus (IAV), which is also widespread in various mammals, including humans and pigs. Influenza B virus (IBV) and Influenza C virus (ICV) pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sympathomimetic Amine
Sympathomimetic drugs (also known as adrenergic drugs and adrenergic amines) are stimulant compounds which mimic the effects of endogenous agonists of the sympathetic nervous system. Examples of sympathomimetic effects include increases in heart rate, force of cardiac contraction, and blood pressure. The primary endogenous agonists of the sympathetic nervous system are the catecholamines (i.e., epinephrine drenaline norepinephrine oradrenaline and dopamine), which function as both neurotransmitters and hormones. Sympathomimetic drugs are used to treat cardiac arrest and low blood pressure, or even delay premature labor, among other things. These drugs can act through several mechanisms, such as directly activating postsynaptic receptors, blocking breakdown and reuptake of certain neurotransmitters, or stimulating production and release of catecholamines. Mechanisms of action The mechanisms of sympathomimetic drugs can be direct-acting (direct interaction between drug and rec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
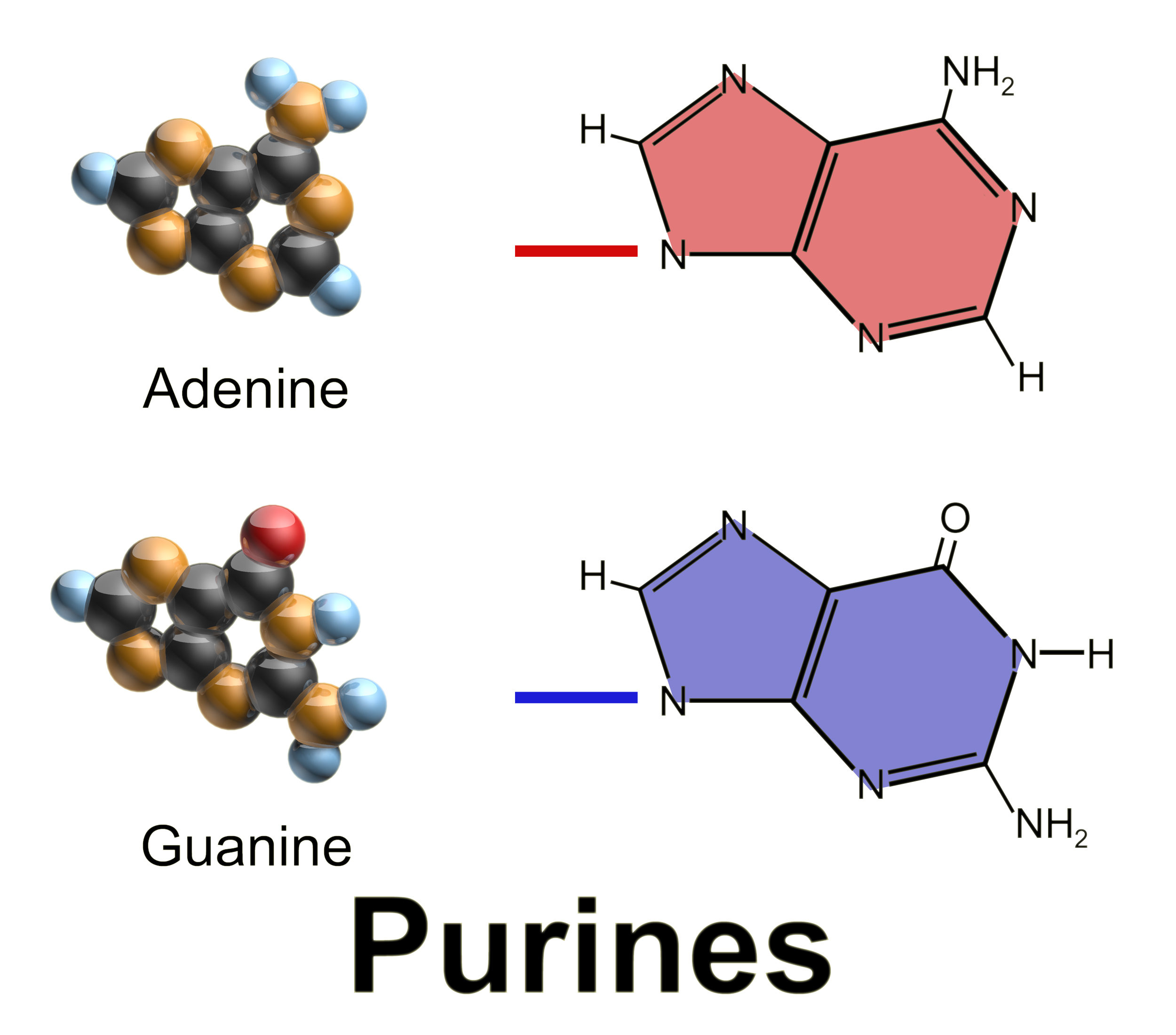
Purine
Purine is a heterocyclic aromatic organic compound that consists of two rings ( pyrimidine and imidazole) fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which include substituted purines and their tautomers. They are the most widely occurring nitrogen-containing heterocycles in nature. Dietary sources Purines are found in high concentration in meat and meat products, especially internal organs such as liver and kidney. In general, plant-based diets are low in purines. High-purine plants and algae include some legumes (lentils and black eye peas) and spirulina. Examples of high-purine sources include: sweetbreads, anchovies, sardines, liver, beef kidneys, brains, meat extracts (e.g., Oxo, Bovril), herring, mackerel, scallops, game meats, yeast (beer, yeast extract, nutritional yeast) and gravy. A moderate amount of purine is also contained in red meat, beef, pork, poultry, fish and seafood, asparagus, cauliflo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |