|
Metastable Isotope
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy excited state, higher energy levels than in the ground state of the same nucleus. "Metastable" describes nuclei whose excited states have Half-life, half-lives 100 to 1000 times longer than the half-lives of the excited nuclear states that decay with a "prompt" half life (ordinarily on the order of 10−12 seconds). The term "metastable" is usually restricted to isomers with half-lives of 10−9 seconds or longer. Some references recommend 5 × 10−9 seconds to distinguish the metastable half life from the normal "prompt" Induced gamma emission, gamma-emission half-life. Occasionally the half-lives are far longer than this and can last minutes, hours, or years. For example, the Isotopes of tantalum#Tantalum-180m, nuclear isomer survives so long (at least 1015 years) that it has never been observed to decay spontaneously. The half-life of a nuclear isomer can eve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metastable
In chemistry and physics, metastability denotes an intermediate Energy level, energetic state within a dynamical system other than the system's ground state, state of least energy. A ball resting in a hollow on a slope is a simple example of metastability. If the ball is only slightly pushed, it will settle back into its hollow, but a stronger push may start the ball rolling down the slope. Bowling pins show similar metastability by either merely wobbling for a moment or tipping over completely. A common example of metastability in science is isomerisation. Higher energy isomers are long lived because they are prevented from rearranging to their preferred ground state by (possibly large) barriers in the potential energy. During a metastable state of finite lifetime, all state-describing parameters reach and hold stationary values. In isolation: *the state of least energy is the only one the system will inhabit for an indefinite length of time, until more external energy is added ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ground State
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. In quantum field theory, the ground state is usually called the vacuum state or the vacuum. If more than one ground state exists, they are said to be degenerate. Many systems have degenerate ground states. Degeneracy occurs whenever there exists a unitary operator that acts non-trivially on a ground state and commutes with the Hamiltonian of the system. According to the third law of thermodynamics, a system at absolute zero temperature exists in its ground state; thus, its entropy is determined by the degeneracy of the ground state. Many systems, such as a perfect crystal lattice, have a unique ground state and therefore have zero entropy at absolute zero. It is also possible for the highest excited state to have absolute zero temper ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angular Momentum
In physics, angular momentum (rarely, moment of momentum or rotational momentum) is the rotational analog of linear momentum. It is an important physical quantity because it is a conserved quantity—the total angular momentum of a closed system remains constant. Angular momentum has both a direction and a magnitude, and both are conserved. Bicycles and motorcycles, frisbees, rifled bullets, and gyroscopes owe their useful properties to conservation of angular momentum. Conservation of angular momentum is also why hurricanes form spirals and neutron stars have high rotational rates. In general, conservation limits the possible motion of a system, but it does not uniquely determine it. The three-dimensional angular momentum for a point particle is classically represented as a pseudovector , the cross product of the particle's position vector (relative to some origin) and its momentum vector; the latter is in Newtonian mechanics. Unlike linear momentum, angular m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Fission Product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release of heat energy (kinetic energy of the nuclei), and gamma rays. The two smaller nuclei are the ''fission products''. (See also Fission products (by element)). About 0.2% to 0.4% of fissions are ternary fissions, producing a third light nucleus such as helium-4 (90%) or tritium (7%). The fission products themselves are usually unstable and therefore radioactive. Due to being relatively neutron-rich for their atomic number, many of them quickly undergo beta decay. This releases additional energy in the form of beta particles, antineutrinos, and gamma rays. Thus, fission events normally result in beta and gamma radiation, even though this radiation is not produced directly by the fission event itself. The produced radionuclides have vary ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Internal Conversion
Internal conversion is a non-radioactive, atomic decay process where an excited nucleus interacts electromagnetically with one of the orbital electrons of an atom. This causes the electron to be emitted (ejected) from the atom. Thus, in internal conversion (often abbreviated IC), a high-energy electron is emitted from the excited atom, but not from the nucleus. For this reason, the high-speed electrons resulting from internal conversion are not called beta particles, since the latter come from beta decay, where they are newly created in the nuclear decay process. IC is possible whenever gamma decay is possible, except if the atom is fully ionized. In IC, the atomic number does not change, and thus there is no transmutation of one element to another. Since an electron is lost from the atom, a hole appears in an electron shell which is subsequently filled by other electrons that descend to that empty, lower energy level, and in the process emit characteristic X-ray(s), Auger elec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Spin
In atomic physics, the spin quantum number is a quantum number (designated ) which describes the intrinsic angular momentum (or spin angular momentum, or simply spin) of an electron or other particle. The phrase was originally used to describe the fourth of a set of quantum numbers (the principal quantum number , the azimuthal quantum number , the magnetic quantum number , and the spin quantum number ), which completely describe the quantum state of an electron in an atom. The name comes from a physical spinning of the electron about an axis, as proposed by Uhlenbeck and Goudsmit. The value of is the component of spin angular momentum parallel to a given direction (the –axis), which can be either +1/2 or –1/2 (in units of the reduced Planck constant). However this simplistic picture was quickly realized to be physically impossible because it would require the electrons to rotate faster than the speed of light. It was therefore replaced by a more abstract quantum-mechanical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
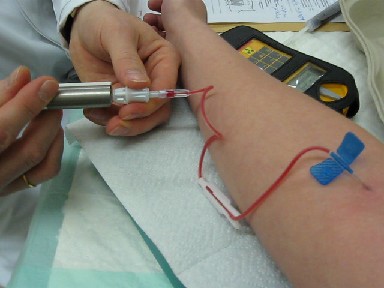
Technetium-99m
Technetium-99m (99mTc) is a metastable nuclear isomer of technetium-99 (itself an isotope of technetium), symbolized as 99mTc, that is used in tens of millions of medical diagnostic procedures annually, making it the most commonly used medical radioisotope in the world. Technetium-99m is used as a radioactive tracer and can be detected in the body by medical equipment (gamma cameras). It is well suited to the role, because it emits readily detectable gamma rays with a photon energy of 140 keV (these 8.8 pm photons are about the same wavelength as emitted by conventional X-ray diagnostic equipment) and its half-life for gamma emission is 6.0058 hours (meaning 93.7% of it decays to 99Tc in 24 hours). The relatively "short" physical half-life of the isotope and its biological half-life of 1 day (in terms of human activity and metabolism) allows for scanning procedures which collect data rapidly but keep total patient radiation exposure low. The same characteristics make the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gamma Ray
A gamma ray, also known as gamma radiation (symbol γ or \gamma), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically shorter than those of X-rays. With frequencies above 30 exahertz (), it imparts the highest photon energy. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900 while studying radiation emitted by radium. In 1903, Ernest Rutherford named this radiation ''gamma rays'' based on their relatively strong penetration of matter; in 1900 he had already named two less penetrating types of decay radiation (discovered by Henri Becquerel) alpha rays and beta rays in ascending order of penetrating power. Gamma rays from radioactive decay are in the energy range from a few kiloelectronvolts (keV) to approximately 8 megaelectronvolts (MeV), corresponding to the typical energy levels in nuclei with reasonably long lif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Binding Energy
Nuclear binding energy in experimental physics is the minimum energy that is required to disassemble the atomic nucleus, nucleus of an atom into its constituent protons and neutrons, known collectively as nucleons. The binding energy for stable nuclei is always a positive number, as the nucleus must gain energy for the nucleons to move apart from each other. Nucleons are attracted to each other by the strong nuclear force. In theoretical nuclear physics, the nuclear binding energy is considered a negative number. In this context it represents the energy of the nucleus relative to the energy of the constituent nucleons when they are infinitely far apart. Both the experimental and theoretical views are equivalent, with slightly different emphasis on what the binding energy means. The mass of an atomic nucleus is less than the sum of the individual masses of the free Wiktionary:constituent, constituent protons and neutrons. The difference in mass can be calculated by the Mass–energ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binding Energy
In physics and chemistry, binding energy is the smallest amount of energy required to remove a particle from a system of particles or to disassemble a system of particles into individual parts. In the former meaning the term is predominantly used in condensed matter physics, atomic physics, and chemistry, whereas in nuclear physics the term ''separation energy'' is used. A bound system is typically at a lower energy level than its unbound constituents. According to relativity theory, a decrease in the total energy of a system is accompanied by a decrease in the total mass, where . Types of binding energy There are several types of binding energy, each operating over a different distance and energy scale. The smaller the size of a bound system, the higher its associated binding energy. Mass–energy relation A bound system is typically at a lower energy level than its unbound constituents because its mass must be less than the total mass of its unbound constituents. For sys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionization Energy
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules and ions, or through the interaction with electromagnetic radiation. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected. Uses Everyday examples of gas ionization are such as within a fluorescent lamp or other electrical discharge lamps. It is also used in radiation detectors such as the Geiger-Müller counter or the ionization chamber. The ionizati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond-dissociation Energy
The bond-dissociation energy (BDE, ''D''0, or ''DH°'') is one measure of the strength of a chemical bond . It can be defined as the standard enthalpy change when is cleaved by homolysis to give fragments A and B, which are usually radical species. The enthalpy change is temperature-dependent, and the bond-dissociation energy is often defined to be the enthalpy change of the homolysis at 0 K (absolute zero), although the enthalpy change at 298 K (standard conditions) is also a frequently encountered parameter. As a typical example, the bond-dissociation energy for one of the C−H bonds in ethane () is defined as the standard enthalpy change of the process : , : ''DH''°298() = Δ''H°'' = 101.1(4) kcal/mol = 423.0 ± 1.7 kJ/mol = 4.40(2) eV (per bond). To convert a molar BDE to the energy needed to dissociate the bond ''per molecule'', the conversion factor 23.060 kcal/mol (96.485 kJ/mol) for each eV can be used. A variety of experim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |