|
Maleic Acid
Maleic acid or ''cis''-butenedioic acid is an organic compound that is a dicarboxylic acid, a molecule with two carboxyl groups. Its chemical formula is HO2CCH=CHCO2H. Maleic acid is the ''cis''-isomer of butenedioic acid, whereas fumaric acid is the ''trans''-isomer. It is mainly used as a precursor to fumaric acid, and relative to its parent maleic anhydride, maleic acid has few applications. Physical properties Maleic acid has a ''heat of combustion'' of -1,355 kJ/mol., 22.7 kJ/mol higher than that of fumaric acid. Maleic acid is more soluble in water than fumaric acid. The melting point of maleic acid (135 °C) is also much lower than that of fumaric acid (287 °C). Both properties of maleic acid can be explained on account of the intramolecular hydrogen bonding that takes place in maleic acid at the expense of intermolecular interactions, and that are not possible in fumaric acid for geometric reasons. Production and industrial applications In industry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They at oftentimes have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid (C3H7CO2H) is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on ano ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
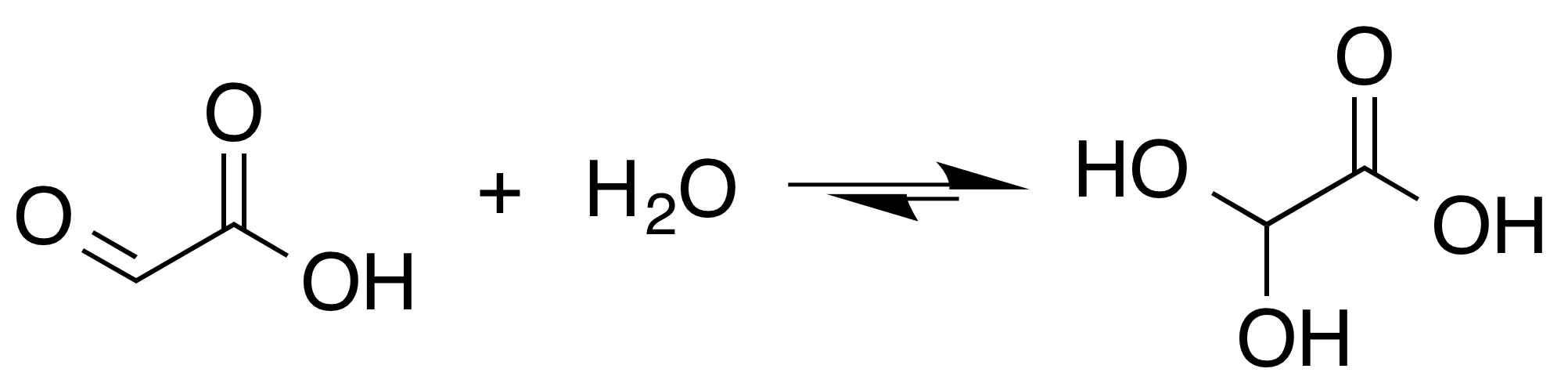
Glyoxylic Acid
Glyoxylic acid or oxoacetic acid is an organic compound. Together with acetic acid, glycolic acid, and oxalic acid, glyoxylic acid is one of the C2 carboxylic acids. It is a colourless solid that occurs naturally and is useful industrially. Structure and nomenclature Although the structure of glyoxylic acid is described as having an aldehyde functional group, the aldehyde is only a minor component of the form most prevalent in some situations. Instead, it often exists as a hydrate or a cyclic dimer. For example, in the presence of water, the carbonyl rapidly converts to a geminal diol (described as the "monohydrate"). The equilibrium constant (''K'') is 300 for the formation of dihydroxyacetic acid at room temperature: : In solution, the monohydrate exists in equilibrium with a hemiacylal dimer form:Georges Mattioda and Yani Christidis “Glyoxylic Acid” Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. : In isolation, the aldehyde structur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maleate Isomerase
In enzymology, a maleate isomerase (), or maleate cis-tran isomerase, is a member of the Asp/Glu racemase superfamily discovered in bacteria. It is responsible for catalyzing cis-trans isomerization of the C2-C3 double bond in maleate to produce fumarate, which is a critical intermediate in citric acid cycle. The presence of an exogenous mercaptan is required for catalysis to happen. : Maleate isomerase participates in butanoate metabolism and nicotinate and nicotinamide metabolism. It is an essential enzyme for the last step of metabolic degradation pathway of nicotinic acid. Recently, maleate isomerase has been an industrial target for degradation of tobacco waste. It is also got attention for its involvement in aspartic acid and maleic acid production. Maleate isomerase has been utilized by multiple bacteria species, including ''Pseudomonas fluorescens'', ''Alcaligenes faecalis'', ''Bacillus stearothermophilus'', ''Serratia marcescens'''', Pseudomonas putida'' and ''Noca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloric Acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid Acid strength is the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a proton, H+, and an anion, A-. The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions .... It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. History In the early tenth century, the Persian physician and alchemist Abu Bakr al-Razi ( 865–925, Latin: Rhazes) conducted experiments with sal ammoniac (ammonium chloride) and vitriol (hydrated sulfates of various metals), which he distilled together, thus producing the gas hydrogen chloride. In doing so, al-Razi may have stumbled upon a primitive method ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radical Addition
In organic chemistry, free-radical addition is an addition reaction which involves free radicals. The addition may occur between a radical and a non-radical, or between two radicals. The basic steps with examples of the free-radical addition (also known as radical chain mechanism) are: * Initiation by a radical initiator: A radical is created from a non-radical precursor. * Chain propagation: A radical reacts with a non-radical to produce a new radical species * Chain termination: Two radicals react with each other to create a non-radical species Free-radical reactions depend on a reagent having a (relatively) weak bond, allowing it to homolyse to form radicals (often with heat or light). Reagents without such a weak bond would likely proceed via a different mechanism. An example of an addition reaction involving aryl radicals is the Meerwein arylation. Addition of mineral acid to an alkene To illustrate, consider the alkoxy radical-catalyzed, anti-Markovnikov reaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Free-radical
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes. A notable example of a radical is the hydroxyl radical (HO·), a molecule that has one unpaired electron on the oxygen atom. Two other examples are triplet oxygen and triplet carbene (꞉) which have two unpaired electrons. Radicals may be generated in a number of ways, but typical methods involve redox reactions. Ionizing radiation, heat, electrical discharges, and electrolysis are known to produce radicals. Radicals are intermediates in many chemical reactions, more so than is apparent from the balanced equations. Radicals are important in combustion, atmospheric chemistry, polymerization, plasma chemistry, biochemistry, and many other chemical processes. A majority o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University Of Regensburg
The University of Regensburg (german: link=no, Universität Regensburg) is a public research university located in the medieval city of Regensburg, Bavaria, a city that is listed as a UNESCO World Heritage Site. The university was founded on 18 July 1962 by the Landtag of Bavaria as the fourth full-fledged university in Bavaria. Following groundbreaking in 1965, the university officially opened to students during the 1967–1968 winter semester, initially housing faculties in Law and Business Sciences and Philosophy. During the summer semester of 1968 the faculty of Theology was created. Currently, the University of Regensburg houses eleven faculties. The university actively participates in the European Union's SOCRATES programme as well as several TEMPUS programmes. Its most famous academic, the previous Pope Benedict XVI, served as a professor there until 1977 and formally retained his chair in theology. History Attempts to establish a university in Regensburg had been advoc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table ( halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig (in 1825) and Antoine Jérôme Balard (in 1826), its name was derived from the Ancient Greek (bromos) meaning "stench", referring to its sharp and pungent smell. Elemental bromine is very reactive and thus does not occur as a native element in nature but it occurs in colourless soluble crystalline mineral halide salts, analogous to table salt. In fact, bromine and all the halogens are so reactive that they form bonds in pairs—never in single atoms. While it is rather rare in the Earth's crust, the high solubility of the bromide ion (Br) has caused its accumulation in the oceans. Comme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photolysis
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons. It is defined as the interaction of one or more photons with one target molecule. Photodissociation is not limited to visible light. Any photon with sufficient energy can affect the chemical bonds of a chemical compound. Since a photon's energy is inversely proportional to its wavelength, electromagnetic radiations with the energy of visible light or higher, such as ultraviolet light, x-rays, and gamma rays can induce such reactions. Photolysis in photosynthesis Photolysis is part of the light-dependent reaction or light phase or photochemical phase or Hill reaction of photosynthesis. The general reaction of photosynthetic photolysis can be given in terms of photons as: :\ce + 2 \text \longrightarrow \ce The chemical nature of "A" depends on the type of organism. Purple sulfur bacteria oxidize hydrogen sulfide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist between two different elements: for example, in a carbonyl group between a carbon atom and an oxygen atom. Other common double bonds are found in azo compounds (N=N), imines (C=N), and sulfoxides (S=O). In a skeletal formula, a double bond is drawn as two parallel lines (=) between the two connected atoms; typographically, the equals sign is used for this. Double bonds were first introduced in chemical notation by Russian chemist Alexander Butlerov. Double bonds involving carbon are stronger and shorter than single bonds. The bond order is two. Double bonds are also electron-rich, which makes them potentially more reactive in the presence of a strong electron acceptor (as in addition reactions of the halogens). File:Ethene structural.svg, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiourea
Thiourea () is an organosulfur compound with the formula and the structure . It is structurally similar to urea (), except that the oxygen atom is replaced by a sulfur atom (as implied by the ''thio-'' prefix); however, the properties of urea and thiourea differ significantly. Thiourea is a reagent in organic synthesis. " Thioureas" refer to a broad class of compounds with the general structure . Thioureas are related to thioamides, e.g. , where R is methyl, ethyl, etc. Structure and bonding Thiourea is a planar molecule. The C=S bond distance is 1.71 Å. The C-N distances average 1.33 Å. The weakening of the C-S bond by C-N pi-bonding is indicated by the short C=S bond in thiobenzophenone, which is 1.63 Å. Thiourea occurs in two tautomeric forms, of which the thione form predominates in aqueous solutions. The equilibrium constant has been calculated as ''K''eq is . The thiol form, which is also known as an isothiourea, can be encountered in substituted compounds such a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mineral Acid
A mineral acid (or inorganic acid) is an acid derived from one or more inorganic compounds, as opposed to organic acids which are acidic, organic compounds. All mineral acids form hydrogen ions and the conjugate base when dissolved in water. Characteristics Commonly used mineral acids are sulfuric acid (H2SO4), hydrochloric acid (HCl) and nitric acid (HNO3, they are also known as bench acids). Mineral acids range from superacids (perchloric acid) to very weak ones (boric acid). Mineral acids tend to be very soluble in water and insoluble in organic solvents. Mineral acids are used in many sectors of the chemical industry as feedstocks for the synthesis of other chemicals, both organic and inorganic. Large quantities of these acids – especially sulfuric acid, nitric acid, and hydrochloric acid – are manufactured for commercial use in large plants. Mineral acids are also used directly for their corrosive properties. For example, a dilute solution of hydrochloric acid is used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |