|
Microwave Spectroscopy
Microwave spectroscopy is the spectroscopy method that employs microwaves, i.e. electromagnetic radiation at GHz frequencies, for the study of matter. History The ammonia molecule NH3 is shaped like a pyramid 0.38 Å in height, with an equilateral triangle of hydrogens forming the base.The nitrogen situated on the axis has two equivalent equilibrium positions above and below the triangle of hydrogens, and this raises the possibility of the nitrogen tunneling up and down, through the plane of the H-atoms. In 1932 Dennison et al. ... analyzed the vibrational energy of this molecule and concluded that the vibrational energy would be split into pairs by the presence of these two equilibrium positions. The next year Wright and Randall observed ... a splitting of 0.67 cm–1 in far infrared lines, corresponding to a frequency of 20 GHz, the value predicted by theory. In 1934 Cleeton and Williams ... constructed a grating echelle spectrometer in order to measure this splitting directl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectroscopy
Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum. Spectroscopy, primarily in the electromagnetic spectrum, is a fundamental exploratory tool in the fields of astronomy, chemistry, materials science, and physics, allowing the composition, physical structure and electronic structure of matter to be investigated at the atomic, molecular and macro scale, and over astronomical distances. Historically, spectroscopy originated as the study of the wavelength dependence of the absorption by gas phase matter of visible light dispersed by a prism. Current applications of spectroscopy include biomedical spectroscopy in the areas of tissue analysis and medical imaging. Matter waves and acoustic waves can also be considered forms of radiative energy, and recently gravitational waves have been associa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
London Penetration Depth
In superconductors, the London penetration depth (usually denoted as \lambda or \lambda_L) characterizes the distance to which a magnetic field penetrates into a superconductor and becomes equal to e^ times that of the magnetic field at the surface of the superconductor. Typical values of λL range from 50 to 500 nm. It was first derived by Geertruida de Haas-Lorentz in 1925, and later by Fritz and Heinz London in their London equations (1935).Fossheim, Kristian, and Asle Sudbø. ''Superconductivity: physics and applications''. John Wiley & Sons, 2005. The London penetration depth results from considering the London equation and Ampère's circuital law. If one considers a superconducting half-space, i.e. superconducting for x>0, and weak external magnetic field B0 applied along ''z'' direction in the empty space ''x''<0, then inside the superconductor the magnetic field is given by |
Molecular Physics
Molecular physics is the study of the physical properties of molecules and molecular dynamics. The field overlaps significantly with physical chemistry, chemical physics, and quantum chemistry. It is often considered as a sub-field of atomic, molecular, and optical physics. Research groups studying molecular physics are typically designated as one of these other fields. Molecular physics addresses phenomena due to both molecular structure and individual atomic processes within molecules. Like atomic physics, it relies on a combination of classical and quantum mechanics to describe interactions between electromagnetic radiation and matter. Experiments in the field often rely heavily on techniques borrowed from atomic physics, such as spectroscopy and scattering. Molecular structure In a molecule, both the electrons and nuclei experience similar-scale forces from the Coulomb interaction. However, the nuclei remain at nearly fixed locations in the molecule while the electrons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
G-factor (physics)
A ''g''-factor (also called ''g'' value) is a dimensionless quantity that characterizes the magnetic moment and angular momentum of an atom, a particle or the nucleus. It is the ratio of the magnetic moment (or, equivalently, the gyromagnetic ratio) of a particle to that expected of a classical particle of the same charge and angular momentum. In nuclear physics, the nuclear magneton replaces the classically expected magnetic moment (or gyromagnetic ratio) in the definition. The two definitions coincide for the proton. Definition Dirac particle The spin magnetic moment of a charged, spin-1/2 particle that does not possess any internal structure (a Dirac particle) is given by \boldsymbol \mu = g \mathbf S , where ''μ'' is the spin magnetic moment of the particle, ''g'' is the ''g''-factor of the particle, ''e'' is the elementary charge, ''m'' is the mass of the particle, and S is the spin angular momentum of the particle (with magnitude ''ħ''/2 for Dirac particles). B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X Band
The X band is the designation for a band of frequencies in the microwave radio region of the electromagnetic spectrum. In some cases, such as in communication engineering, the frequency range of the X band is set at approximately 7.0–11.2 GHz. In radar engineering, the frequency range is specified by the Institute of Electrical and Electronics Engineers (IEEE) as 8.0–12.0 GHz. The X band is used for radar, satellite communication, and wireless computer networks. Radar X band is used in radar applications, including continuous-wave, pulsed, single- polarization, dual-polarization, synthetic aperture radar, and phased arrays. X-band radar frequency sub-bands are used in civil, military, and government institutions for weather monitoring, air traffic control, maritime vessel traffic control, defense tracking, and vehicle speed detection for law enforcement. X band is often used in modern radars. The shorter wavelengths of the X band provide higher-r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zeeman Effect
The Zeeman effect () is the splitting of a spectral line into several components in the presence of a static magnetic field. It is caused by the interaction of the magnetic field with the magnetic moment of the atomic electron associated with its Angular momentum, orbital motion and Spin (physics), spin; this interaction shifts some orbital energies more than others, resulting in the split spectrum. The effect is named after the Netherlands, Dutch physicist Pieter Zeeman, who discovered it in 1896 and received a Nobel Prize in Physics for this discovery. It is analogous to the Stark effect, the splitting of a spectral line into several components in the presence of an electric field. Also, similar to the Stark effect, transitions between different components have, in general, different intensities, with some being entirely forbidden (in the dipole approximation), as governed by the selection rules. Since the distance between the Zeeman sub-levels is a function of magnetic field ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferromagnetic Resonance
Ferromagnetic resonance, or FMR, is coupling between an electromagnetic wave and the magnetization of a medium through which it passes. This coupling induces a significant loss of power of the wave. The power is absorbed by the precessing magnetization (Larmor precession) of the material and lost as heat. For this coupling to occur, the frequency of the incident wave must be equal to the precession frequency of the magnetization (Larmor frequency) and the polarization of the wave must match the orientation of the magnetization. This effect can be used for various applications such as spectroscopic techniques or conception of microwave devices. The FMR spectroscopic technique is used to probe the magnetization of ferromagnetic materials. It is a standard tool for probing spin waves and spin dynamics. FMR is very broadly similar to electron paramagnetic resonance (EPR), and also somewhat similar to nuclear magnetic resonance (NMR), except that FMR probes the sample magnetizati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Spin Resonance
Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the spins excited are those of the electrons instead of the atomic nuclei. EPR spectroscopy is particularly useful for studying metal complexes and organic radicals. EPR was first observed in Kazan State University by Soviet physicist Yevgeny Zavoisky in 1944, and was developed independently at the same time by Brebis Bleaney at the University of Oxford. Theory Origin of an EPR signal Every electron has a magnetic moment and spin quantum number s = \tfrac , with magnetic components m_\mathrm = + \tfrac or m_\mathrm = - \tfrac . In the presence of an external magnetic field with strength B_\mathrm , the electron's magnetic moment aligns itself either antiparallel ( m_\mathrm = - \tfrac ) or parallel ( m_\mathrm = + \tfrac ) to the fie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spin Magnetic Moment
Spin is an intrinsic form of angular momentum carried by elementary particles, and thus by composite particles such as hadrons, atomic nuclei, and atoms. Spin is quantized, and accurate models for the interaction with spin require relativistic quantum mechanics or quantum field theory. The existence of electron spin angular momentum is inferred from experiments, such as the Stern–Gerlach experiment, in which silver atoms were observed to possess two possible discrete angular momenta despite having no orbital angular momentum. The relativistic spin–statistics theorem connects electron spin quantization to the Pauli exclusion principle: observations of exclusion imply half-integer spin, and observations of half-integer spin imply exclusion. Spin is described mathematically as a vector for some particles such as photons, and as a spinor or bispinor for other particles such as electrons. Spinors and bispinors behave similarly to vectors: they have definite magnitudes and c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drude Model
The Drude model of electrical conduction was proposed in 1900 by Paul Drude to explain the transport properties of electrons in materials (especially metals). Basically, Ohm's law was well established and stated that the current and voltage driving the current are related to the resistance of the material. The inverse of the resistance is known as the conductance. When we consider a metal of unit length and unit cross sectional area, the conductance is known as the conductivity, which is the inverse of resistivity. The Drude model attempts to explain the resistivity of a conductor in terms of the scattering of electrons (the carriers of electricity) by the relatively immobile ions in the metal that act like obstructions to the flow of electrons. The model, which is an application of kinetic theory, assumes that the microscopic behaviour of electrons in a solid may be treated classically and behaves much like a pinball machine, with a sea of constantly jittering electrons b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heavy Fermion Material
In materials science, heavy fermion materials are a specific type of intermetallic compound, containing elements with 4f or 5f electrons in unfilled electron bands. Electrons are one type of fermion, and when they are found in such materials, they are sometimes referred to as heavy electrons. Heavy fermion materials have a low-temperature specific heat whose linear term is up to 1000 times larger than the value expected from the free electron model. The properties of the heavy fermion compounds often derive from the partly filled f-orbitals of rare-earth or actinide ions, which behave like localized magnetic moments. The name "heavy fermion" comes from the fact that the fermion behaves as if it has an effective mass greater than its rest mass. In the case of electrons, below a characteristic temperature (typically 10 K), the conduction electrons in these metallic compounds behave as if they had an effective mass up to 1000 times the free particle mass. This large effective ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
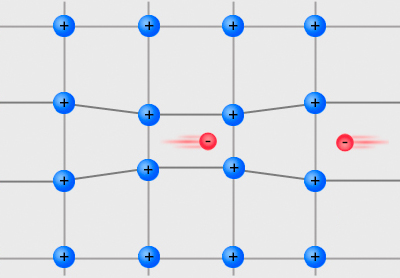
Cooper Pair
In condensed matter physics, a Cooper pair or BCS pair (Bardeen–Cooper–Schrieffer pair) is a pair of electrons (or other fermions) bound together at low temperatures in a certain manner first described in 1956 by American physicist Leon Cooper. Description Cooper showed that an arbitrarily small attraction between electrons in a metal can cause a paired state of electrons to have a lower energy than the Fermi energy, which implies that the pair is bound. In conventional superconductors, this attraction is due to the electron–phonon interaction. The Cooper pair state is responsible for superconductivity, as described in the BCS theory developed by John Bardeen, Leon Cooper, and John Schrieffer for which they shared the 1972 Nobel Prize in Physics. Although Cooper pairing is a quantum effect, the reason for the pairing can be seen from a simplified classical explanation. An electron in a metal normally behaves as a free particle. The electron is repelled from other electrons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |