|
Methylenetriphenylphosphorane
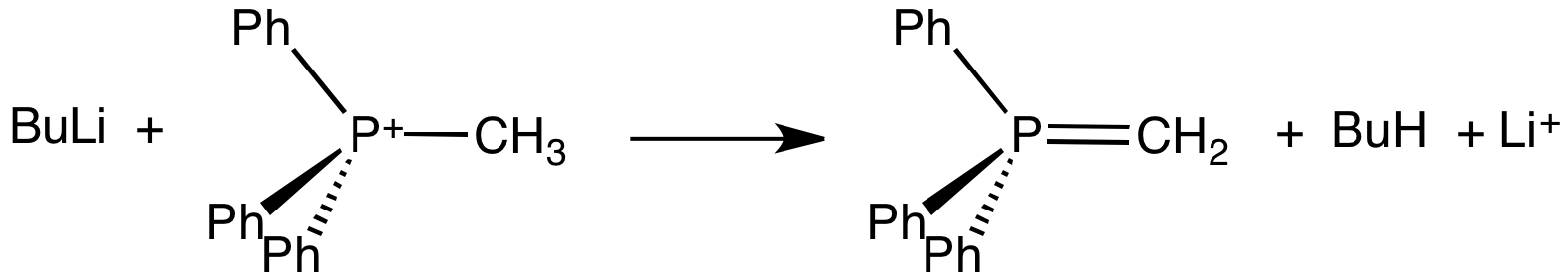
Methylenetriphenylphosphorane is an organophosphorus compound with the formula Ph3PCH2. It is the parent member of the phosphorus ylides, popularly known as Wittig reagents. It is a highly polar, highly basic species. Preparation and use Methylenetriphenylphosphorane is prepared from methyltriphenylphosphonium bromide by its deprotonation using a strong base like butyllithium: :Ph3PCH3Br + BuLi → Ph3PCH2 + LiBr + BuH The phosphorane is generally not isolated, instead it is used in situ. The estimated pKa of this carbon acid is near 15. Potassium tert-butoxide has been used in place of Butyllithium, butyl lithium. Sodium amide has also been used a base. Methylenetriphenylphosphorane is used to replace oxygen centres in Aldehyde, aldehydes and Ketone, ketones with a methylene group, i.e., a methylenation: :R2CO + Ph3PCH2 → R2C=CH2 + Ph3PO The phosphorus-containing product is triphenylphosphine oxide. Structure Crystallographic characterization of the colourless ylide reveals th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbomethoxymethylenetriphenylphosphorane
Carbomethoxymethylenetriphenylphosphorane is a Chemical substance, chemical compound used in Organic compound, organic syntheses. It contains a phosphorus atom bound to three phenyl groups, and doubly bound to the alpha position of methyl acetate. It undergoes a Wittig reaction. It is used in the Vitamin B12 total synthesis. Production Carbomethoxymethylenetriphenylphosphorane can be made via a multistep reaction using bromoacetic acid, N,N'-Dicyclohexylcarbodiimide, dicyclohexylcarbodiimide, and triphenylphosphine. This makes a phosphonium salt, which is converted to the final product by sodium carbonate in water. Reactions Carbomethoxymethylenetriphenylphosphorane reacts with aldehydes to give a two carbon atom extension. The carbomethoxymethylene group replaces the oxygen of the aldehyde to give a ''trans-'' double bond. References Organophosphorus compounds Esters Phenyl compounds Organic compounds {{Organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wittig Reagent
In organic chemistry, Wittig reagents are organophosphorus compounds of the formula R3P=CHR', where R is usually phenyl. They are used to convert ketones and aldehydes to alkenes: : Preparation Because they typically hydrolyze and oxidize readily, Wittig reagents are prepared using air-free techniques. They are typically generated and used in situ. THF is a typical solvent. Some are sufficiently stable to be sold commercially. Formation of phosphonium salt Wittig reagents are usually prepared from a phosphonium salt, which is in turn prepared by the quaternization of triphenylphosphine with an alkyl halide. Wittig reagents are usually derived from a primary alkyl halide. Quaternization of triphenylphosphine with secondary halides is typically inefficient. For this reason, Wittig reagents are rarely used to prepare tetrasubstituted alkenes. Bases for deprotonation of phosphonium salts The alkylphosphonium salt is deprotonated with a strong base such as ''n''-butyllithium: : ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylenation
In organic chemistry, methylenation is a chemical reaction that inserts a methylene () group into a chemical compound: :\ce \longrightarrow \ce\ce In a related sense, it also describes a process in which a divalent group of a starting material is removed and replaced with a terminal CH2 group: : \ce \longrightarrow \ce Methylenation in this context is also known as methenylation. Most commonly, E is an oxygen atom, so that the reaction results in terminal alkenes from aldehydes and ketones, or more rarely, enol ethers from esters or enamines from amides. Methods Methylene Insertion into Alkanes Singlet methylene (1 CH2, produced from photolysis of diazomethane under ultraviolet irradiation, methylenates hydrocarbons. Arenes and olefins undergo methylenation to give cyclopropanated products. In the case of arenes, the cyclopropanation product undergoes further electrocyclic ring opening to give cycloheptatriene products ( Buchner ring expansion). Alkenes undergo bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyltriphenylphosphonium Bromide
Methyltriphenylphosphonium bromide is the organophosphorus compound with the formula C6H5)3PCH3r. It is the bromide salt of a phosphonium cation. It is a white salt that is soluble in polar organic solvents. Synthesis and reactions Methyltriphenylphosphonium bromide is produced by treating triphenylphosphine with methyl bromide: :Ph3P + CH3Br → Ph3PCH3Br Methyltriphenylphosphonium bromide is the principal precursor to methylenetriphenylphosphorane Methylenetriphenylphosphorane is an organophosphorus compound with the formula Ph3PCH2. It is the parent member of the phosphorus ylides, popularly known as Wittig reagents. It is a highly polar, highly basic species. Preparation and use Methylene ..., a useful methylenating reagent. This conversion is achieved by treating methyltriphenylphosphonium bromide with strong base.{{cite journal, doi = 10.1080/00397918508063883, title = The Wittig Reaction Using Potassium-tert-butoxide High Yield Methylenations of Sterically Hindered K ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methoxymethylenetriphenylphosphine
Methoxymethylenetriphenylphosphorane is a Wittig reagent used for the homologization of aldehydes, and ketones to extended aldehydes, an organic reaction first reported in 1958. The reagent is generally prepared and used in situ. It has blood-red color, indicative of destabilized ylides. Preparation The reagent can be prepared in two steps from triphenylphosphine. The first step is ''P''-alkylation with chloromethyl methyl ether. : In the second step, the resulting phosphonium salt is deprotonated. : In place of chloromethyl methyl ether, a mixture of methylal and acetyl chloride can be used. Uses This reagent reacts with a ketone or aldehyde in a Wittig reaction to give an enol ether, which can be converted to the aldehyde by acid-induced hydrolysis. The initial report of the reaction demonstrated its use on the steroid A steroid is an organic compound with four fused compound, fused rings (designated A, B, C, and D) arranged in a specific molecular configuration. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
(Chloromethylene)triphenylphosphorane
(Chloromethylene)triphenylphosphorane is the organophosphorus compound with the formula Ph3P=CHCl (Ph = phenyl). It is a white solid but is usually generated and used in situ as a reagent in organic synthesis. It is structurally and chemically related to methylenetriphenylphosphorane. The reagent is prepared from the chloromethylphosphonium salt h3PCH2Cll by treatment with strong base. The phosphonium compound is generated by treatment of triphenylphosphine with chloroiodomethane. (Chloromethylene)triphenylphosphorane converts aldehydes to vinyl chlorides: :RCHO + Ph3P=CHCl → RCH=CHCl + Ph3PO These vinyl chlorides undergo dehydrochlorination to give alkyne \ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...s: :RCH=CHCl + NaN(SiMe3)2 → RC≡CH + NaCl + HN( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ylide
An ylide () or ylid () is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms have full octets of electrons. The result can be viewed as a structure in which two adjacent atoms are connected by both a covalent and an ionic bond; normally written X+–Y−. Ylides are thus 1,2- dipolar compounds, and a subclass of zwitterions. They appear in organic chemistry as reagents or reactive intermediates. The class name "ylide" for the compound should not be confused with the suffix "-ylide". Resonance structures Many ylides may be depicted by a multiply bonded form in a resonance structure, known as the ylene form, while the actual structure lies in between both forms: : The actual bonding picture of these types of ylides is strictly zwitterionic (the structure on the right) with the strong Coulombic attractio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Resonance Structure
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or ''canonical structures'') into a resonance hybrid (or ''hybrid structure'') in valence bond theory. It has particular value for analyzing delocalized electrons where the bonding cannot be expressed by one single Lewis structure. The resonance hybrid is the accurate structure for a molecule or ion; it is an average of the theoretical (or hypothetical) contributing structures. Overview Under the framework of valence bond theory, resonance is an extension of the idea that the bonding in a chemical species can be described by a Lewis structure. For many chemical species, a single Lewis structure, consisting of atoms obeying the octet rule, possibly bearing formal charges, and connected by bonds of positive integer order, is sufficient for des ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus(V) Compounds
Phosphorus is a chemical element; it has symbol P and atomic number 15. All elemental forms of phosphorus are highly reactive and are therefore never found in nature. They can nevertheless be prepared artificially, the two most common allotropes being white phosphorus and red phosphorus. With as its only stable isotope, phosphorus has an occurrence in Earth's crust of about 0.1%, generally as phosphate rock. A member of the pnictogen family, phosphorus readily forms a wide variety of organic and inorganic compounds, with as its main oxidation states +5, +3 and −3. The isolation of white phosphorus in 1669 by Hennig Brand marked the scientific community's first discovery since Antiquity of an element. The name phosphorus is a reference to the god of the Morning star in Greek mythology, inspired by the faint glow of white phosphorus when exposed to oxygen. This property is also at the origin of the term ''phosphorescence'', meaning glow after illumination, although white pho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quaternary Phosphonium Compounds
The Quaternary ( ) is the current and most recent of the three periods of the Cenozoic Era in the geologic time scale of the International Commission on Stratigraphy (ICS), as well as the current and most recent of the twelve periods of the Phanerozoic eon. It follows the Neogene Period and spans from 2.58 million years ago to the present. The Quaternary Period is divided into two epochs: the Pleistocene (2.58 million years ago to 11.7 thousand years ago) and the Holocene (11.7 thousand years ago to today); a proposed third epoch, the Anthropocene, was rejected in 2024 by IUGS, the governing body of the ICS. The Quaternary is typically defined by the Quaternary glaciation, the cyclic growth and decay of continental ice sheets related to the Milankovitch cycles and the associated climate and environmental changes that they caused. Research history In 1759 Giovanni Arduino proposed that the geological strata of northern Italy could be divided into four successive fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphorus Compounds
Organophosphorus chemistry is the scientific study of the synthesis and properties of organophosphorus compounds, which are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX (nerve agent), VX nerve agents. Phosphorus, like nitrogen, is in pnictogen, group 15 of the periodic table, and thus phosphorus compounds and nitrogen compounds have many similar properties. The definition of organophosphorus compounds is variable, which can lead to confusion. In industrial and environmental chemistry, an organophosphorus compound need contain only an organic substituent, but need not have a direct phosphorus-carbon (P-C) bond. Thus a large proportion of pesticides (e.g., malathion), are often included in this class of compounds. Phosphorus can adopt a v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chem , a place in Finland
{{disambig ...
Chem may refer to: *Chemistry *Chemical * ''Chem'' (journal), a scientific journal published by Cell Press *Post apocalyptic slang for "drugs", medicinal or otherwise in the Fallout video game series. In Ancient Egyptian usage: * ''Khem'' (also spelt ''Chem''), the Egyptian word for "black" *Min (god), in the past erroneously named ''Khem'' CHEM may refer to : *A metabolic panel: for instance, CHEM-7, which is the basic metabolic panel * CHEM-DT, a Canadian television channel See also * Chemo (other) * Kem (other) *Kemi Kemi (; ; ; ) is a cities of Finland, town and municipalities of Finland, municipality of Finland. It is located approximately from the city of Tornio and the Finland–Sweden border, Swedish border. The distance to Oulu is to the south and t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |