|
Methylcyclohexane
Methylcyclohexane (cyclohexylmethane) is an organic compound with the molecular formula is CH3C6H11. Classified as saturated hydrocarbon, it is a colourless liquid with a faint odor. Methylcyclohexane is used as a solvent. It is mainly converted in naphtha reformers to toluene.M. Larry Campbell. "Cyclohexane" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2012. A special use is in PF-1 priming fluid in cruise missiles to aid engine start-up when they run on special nonvolatile jet fuel like JP-10. Methylcyclohexane is also used in some correction fluids (such as White-Out) as a solvent. History While researching hydrogenation of arenes with hydroiodic acid in 1876 as part of his doctoral dissertation, first prepared the hydrocarbon from toluene. He determined its boiling point to be 97°C, its density at 20°C to by 0.76 g/cc and named it hexahydrotoluene. It was soon identified in oil from Baku and obtained by other synthetic methods. Production ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
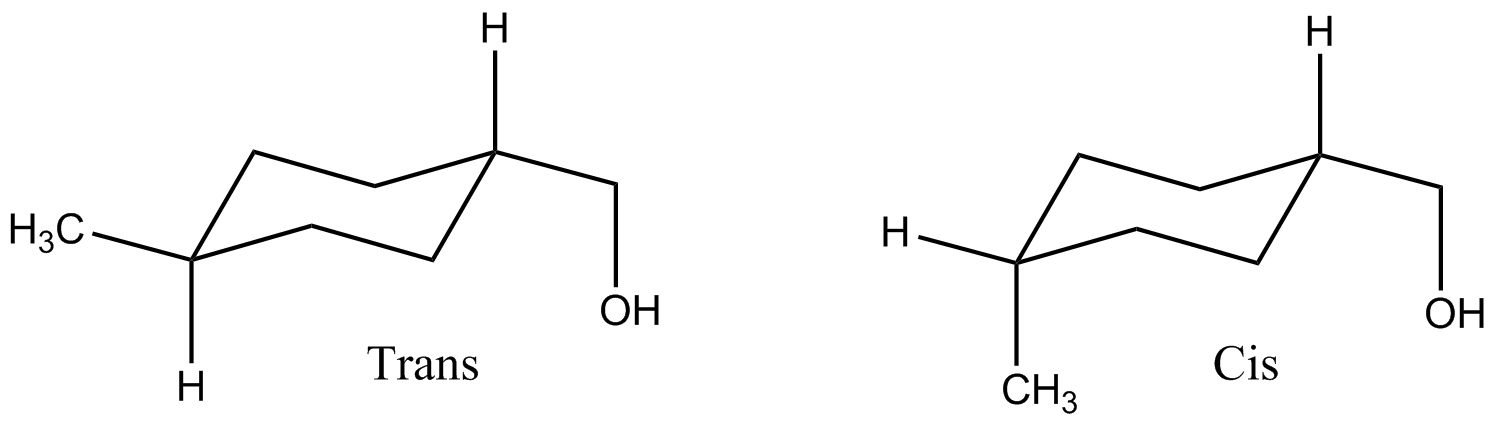
4-methylcyclohexanemethanol
4-Methylcyclohexanemethanol (MCHM, systematic name 4-methylcyclohexylmethanol) is an organic compound with the formula CH3C6H10CH2OH. Classified as a saturated higher alicyclic primary Alcohol (chemistry), alcohol. Both Cis–trans isomerism, cis and trans isomers exist, depending on the relative positions of the methyl (CH3) and hydroxymethyl (CH2OH) groups on the cyclohexane ring. Commercial samples of MCHM consists of a mixture of these isomers as well as other components that vary with the supplier. It is a colourless oil with a faint mint-like alcohol odor. The ''trans'' isomer has a particularly low odor threshold (~7 ppb in water) and a more licorice-like quality which is not associated with the less detectable ''cis'' isomer. Like other 8-carbon alcohols, such as 1-octanol, this compound is only slightly soluble in water but highly soluble in many organic solvents. The solubility of 1-octanol in water is 2.3 grams per liter. Synthesis and production It was first pre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexane Conformation
Cyclohexane conformations are any of several three-dimensional shapes adopted by cyclohexane. Because many compounds feature structurally similar six-membered rings, the structure and dynamics of cyclohexane are important prototypes of a wide range of compounds. The internal angles of a regular, flat hexagon are 120°, while the preferred angle between successive bonds in a carbon chain is about 109.5°, the tetrahedral angle (the arc cosine of −). Therefore, the cyclohexane ring tends to assume non-planar (warped) conformations, which have all angles closer to 109.5° and therefore a lower strain energy than the flat hexagonal shape. Consider the carbon atoms numbered from 1 to 6 around the ring. If we hold carbon atoms 1, 2, and 3 stationary, with the correct bond lengths and the tetrahedral angle between the two bonds, and then continue by adding carbon atoms 4, 5, and 6 with the correct bond length and the tetrahedral angle, we can vary the three dihedral angles f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
JP-10 (fuel)
JP-10 (Jet Propellant 10) is a synthetic jet fuel, specified and used mainly as fuel in missiles. Being designed for military purposes, it is not a kerosene based fuel. Developed to be a gas turbine fuel for cruise missiles, it contains mainly exo-tetrahydrodicyclopentadiene (exo-THDCPD) with some endo-isomer impurity. About 100 ppm of alkylphenol-based antioxidant is added to prevent gumming. Optionally, 0.10–0.15% of fuel system icing inhibitor may be added. Exo-THDCPD is produced by catalytic hydrogenation of dicyclopentadiene and then isomerization. It superseded JP-9, which is a mixture of norbornadiene-based RJ-5 fuel, tetrahydrodicyclopentadiene and methylcyclohexane, because of a lower temperature service limit and about four times lower price. Since the lack of volatile methylcyclohexane makes its ignition difficult, a separate priming fluid PF-1 with about 10-12% of this additive is required for the engine start-up. Its main use is in the Tomahawk missiles. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon with the chemical formula , often abbreviated as , where Ph stands for the phenyl group. It is a colorless, water Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...-insoluble liquid with the odor associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) attached to a phenyl group by a single bond. As such, its systematic IUPAC nomenclature of organic chemistry, IUPAC name is methylbenzene. Toluene is predominantly used as an industrial feedstock and a solvent. As the solvent in some types of paint thinner, permanent markers, contact cement and certain types of glue, toluene is sometimes used as a recreational inhalant and has the potential of causin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalytic Reformer
Catalytic reforming is a chemical process used to convert naphthas from crude oil into liquid products called reformates, which are premium "blending stocks" for high-octane gasoline. The process converts low-octane linear hydrocarbons (paraffins) into branched alkanes (isoparaffins) and cyclic naphthenes, which are then partially dehydrogenated to produce high-octane aromatic hydrocarbons. The dehydrogenation also produces significant amounts of byproduct hydrogen gas, which is fed into other refinery processes such as hydrocracking. A side reaction is hydrogenolysis, which produces light hydrocarbons of lower value, such as methane, ethane, propane and butanes. In addition to a gasoline blending stock, reformate is the main source of aromatic bulk chemicals such as benzene, toluene, xylene and ethylbenzene, which have diverse uses, most importantly as raw materials for conversion into plastics. However, the benzene content of reformate makes it carcinogenic, which has led to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strain (chemistry)
In chemistry, a molecule experiences strain when its chemical structure undergoes some Stress (mechanics), stress which raises its internal energy in comparison to a strain-free reference Chemical compound, compound. The internal energy of a molecule consists of all the energy stored within it. A strained molecule has an additional amount of internal energy which an unstrained molecule does not. This extra internal energy, or strain energy, can be likened to a compression (physics), compressed spring (device), spring.Anslyn and Dougherty, ''Modern Physical Organic Chemistry'', University Science Books, 2006, Much like a compressed spring must be held in place to prevent release of its potential energy, a molecule can be held in an energetically unfavorable conformation by the Chemical bond, bonds within that molecule. Without the bonds holding the conformation in place, the strain energy would be released. Summary Thermodynamics The Chemical equilibrium, equilibrium of two c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jet Fuel Surrogate
Fuel surrogates are mixtures of one or more simple fuels that are designed to emulate either the physical properties (vapor pressure) or combustion properties ( laminar flame speed, heating value, etc.) of a more complex fuel. While surrogate mixtures can demonstrate more than one characteristic of the desired fuel, more often than not different components are required in order to emulate the wide variety of properties that are of interest to researchers. Jet fuel is an example of a fuel requiring a surrogate for experimental research and numerical modelling due to its complexity and high content variability from one batch to the next. Neat hydrocarbon jet fuel surrogate components include decane, dodecane, methylcyclohexane, and toluene. Gasoline surrogate components include n-heptane and iso-octane. Hexadecane is a diesel surrogate component. Biodiesel surrogate components include methyl butyrate Methyl butyrate, also known under the systematic name methyl butanoate, is the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heptane
Heptane or ''n''-heptane is the straight-chain alkane with the chemical formula H3C(CH2)5CH3 or C7H16. When used as a test fuel component in anti-knock test engines, a 100% heptane fuel is the zero point of the octane rating scale (the 100 point is 100% iso-octane). Octane number equates to the anti-knock qualities of a comparison mixture of heptane and iso-octane which is expressed as the percentage of iso-octane in heptane, and is listed on pumps for gasoline (petrol) dispensed globally. History Normal heptane was discovered in 1862 by Carl Schorlemmer, who, while analyzing pyrolysis products of the cannel coal mined in Wigan, identified, separated by fractional distillation and studied a series of liquid hydrocarbons inert to nitric and sulfuric acids. One of them, which he called hydride of heptyl (oenanthyl), had an empirical formula of C7H16, density of 0.709 at 18 °C and boiled between 98 and 99 °C. In the next year he identified the same compound in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colourless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexane is mainly used for the industrial production of adipic acid and caprolactam, which are Precursor (chemistry), precursors to nylon. Cyclohexyl () is the alkyl substituent of cyclohexane and is abbreviated Cy. Production Cyclohexane is one of components of naphtha, from which it can be extracted by advanced distillation methods. Distillation is usually combined with isomerization of methylcyclopentane, a similar component extracted from naphtha by similar methods. Together, these processes cover only a minority (15-20%) of the modern industrial demand, and are complemented by synthesis. Modern industrial synthesis On an industrial scale, cyclohexane is produced by hydrogenation of benzene in the presence of a Raney nickel catalyst. Prod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Group
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bonded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For ex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly Combustibility and flammability, flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a Precursor (chemistry), precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major Chemical industry, industrial che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |