|
Mesoionic
In chemistry, mesoionic compounds are one in which a heterocyclic structure is dipolar and where both the negative and the positive charges are delocalized. A completely uncharged structure cannot be written and mesoionic compounds cannot be represented satisfactorily by any one mesomeric structure. Mesoionic compounds are a subclass of betaines. Examples are sydnones and sydnone imines (e.g. the stimulant mesocarb), münchnones, and mesoionic carbenes. The formal positive charge is associated with the ring atoms and the formal negative charge is associated either with ring atoms or an exocyclic nitrogen or other atom. These compounds are stable zwitterionic compounds and belong to nonbenzenoid aromatic In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...s. See also * Mesom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mesoionic Carbene
In chemistry, mesoionic carbenes (MICs) are a type of reactive intermediate that are related to N-heterocyclic carbenes (NHCs); thus, MICs are also referred to as abnormal N-heterocyclic carbenes (aNHCs) or remote N-heterocyclic carbenes (rNHCs). Unlike simple NHCs, the canonical resonance structures of these carbenes are mesoionic: an MIC cannot be drawn without adding additional charges to some of the atoms. A variety of free carbenes can be isolated and are stable at room temperature. Other free carbenes are not stable and are susceptible to intermolecular decomposition pathways. MICs do not dimerize according to Wanzlick equilibrium as do normal NHCs. This results in relaxed steric requirements for mesoionic carbenes as compared to NHCs.G. Guisado-Barrios, J. Bouffard, B. Donnadieu, G. Bertrand. ''Angew. Chem., Int. Ed''. 2010, 49, 4759-4762.D. Martin, M. Melaimi, M. Soleilhavoup, G. Bertrand. ''Organometallics''. 2011, 30, 5304-5313.G. Ung, D. Mendoza-Espinosa, J. Bouf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
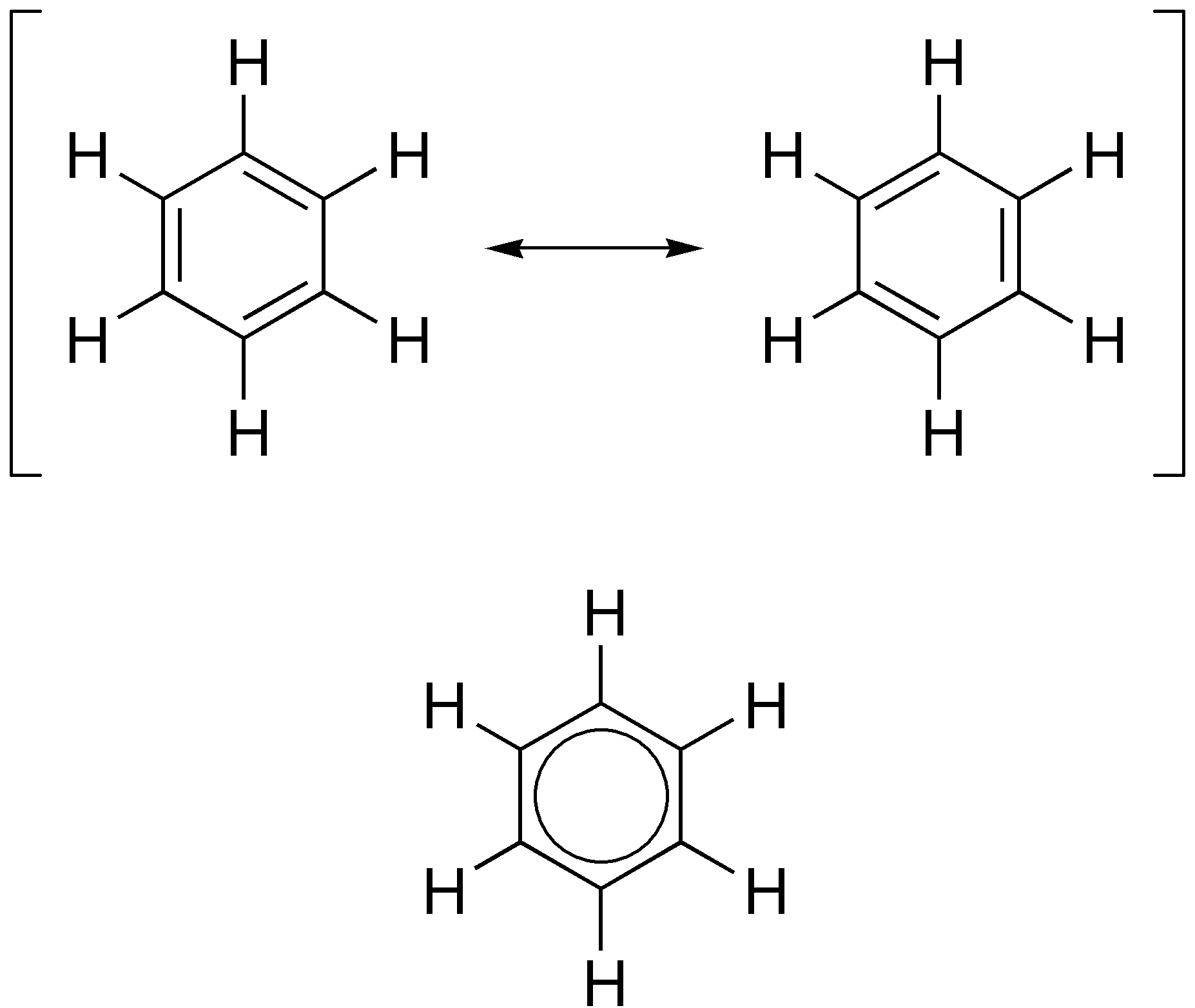
Sydnone Structures
Sydnones are mesoionic heterocyclic chemical compounds possessing a C5-oxygenated 1,2,3-oxadiazole core, named after the city of Sydney, Australia. Like other mesoionic compounds they are dipolar, possessing both positive and negative charges which are delocalized across the ring. Discovery N-phenylsydnone was first prepared in 1935 by and by cyclodehydration of N-Nitroso- N-phenylglycine with acetic anhydride. Later work showed that this could be applied fairly generally to the nitrosamines of N-substituted amino acids. The parent compound sydnone is not synthetically accessible and may not exist. Chemical structure Sydnones have the following resonance structures. The exocyclic oxygen atom (O6) has a significant negative charge. Recent computational studies have indicated that sydnones and other similar mesoionic compounds are nonaromatic, "though well-stabilized in two separate regions by electron and charge delocalization." Examples * Cefanone ( Cephanone) * Ipra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mesomeric Betaine
Mesomeric betaines are dipolar heterocyclic compounds in which both the negative and the positive charges are delocalized. Examples are mesoionic compounds and heteropentalenes (e.g. diazapentalenes). Heteropentalenes are not mesoionic In chemistry, mesoionic compounds are one in which a heterocyclic structure is dipolar and where both the negative and the positive charges are delocalized. A completely uncharged structure cannot be written and mesoionic compounds cannot be repr .... References Heterocyclic compounds Aromatic compounds {{aromatic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sydnone Imine
{{Short description, Class of chemical compounds Sydnone imine is a mesoionic heterocyclic aromatic chemical compound. Sydnone imine is the imine of sydnone where the keto functional group of sydnone (=O) has been replaced with an imine (=NH) group. Pharmaceutical drugs A variety of pharmaceutical drugs include sydnone imines in their chemical structure including feprosidnine, linsidomine, mesocarb, and molsidomine, among others. Chemical structure In sydnone imines, both the negative and the positive charges are delocalized within the ring and the imine group. : See also * Montréalone * Münchnone * Sydnone Sydnones are mesoionic heterocyclic chemical compounds possessing a C5-oxygenated 1,2,3-oxadiazole core, named after the city of Sydney, Australia. Like other mesoionic compounds they are dipolar, possessing both positive and negative charges wh ... External links IUPAC Goldbook entryDictionary of Organic Compounds Simple aromatic rings Oxadiazoles Imines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Münchnone
Münchnone (synonyms: 1,3-oxazolium-5-oxide; 1,3-oxazolium-5-olate; anhydro-5-hydroxy-1,3-oxazolium hydroxide; 5-hydroxy-1,3-oxazolium hydroxide, inner salt; oxido-oxazolium) is a mesoionic heterocyclic compound, heterocyclic aromatic chemical compound, with the molecular formula C3H3NO2. The name refers to the city of Munich, Germany (), where the compound and its derivatives were first discovered and studied. Synthesis and reactivity The first preparation of a münchnone derivative was reported in 1959 by Lawson & Miles by cyclodehydration of 2-pyridone-''N''-acetic acid with acetic anhydride. The azomethine ylide reactivity of münchnones, and their reaction with alkynes in the synthesis of pyrroles, was first published by Rolf Huisgen, Huisgen et al. The Huisgen group followed up with a thorough investigation of the chemical properties, reactivity, and utility of münchnones towards the synthesis of many other products. As such, they are typically credited for the discovery o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mesocarb
Mesocarb, sold under the brand name Sidnocarb or Sydnocarb and known by the developmental code name MLR-1017, is a psychostimulant medication which has been used in the treatment of psychiatric disorders and for a number of other indications in the Soviet Union and Russia. It is currently under development for the treatment of Parkinson's disease and sleep disorders. It is taken by mouth. The drug is a selective dopamine reuptake inhibitor (DRI). It is an unusual and unique DRI, acting as a negative allosteric modulator and non-competitive inhibitor of the dopamine transporter (DAT). Chemically, mesocarb contains amphetamine within its structure but has been modified and extended at the amine with a sydnone imine-containing moiety. Mesocarb was first described by 1971. It was used as a pharmaceutical drug until 2008. In 2021, its nature as a DAT allosteric modulator was reported. As of February 2023, mesocarb was in phase 1 clinical trials for Parkinson's disease. The act ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugation alone. The earliest use of the term was in an article by August Wilhelm Hofmann in 1855. There is no general relationship between aromaticity as a chemical property and the olfaction, olfactory properties of such compounds. Aromaticity can also be considered a manifestation of cyclic delocalization and of Resonance (chemistry), resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-covalent bond, bonded to one another. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by Friedrich August Kekulé ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zwitterion
In chemistry, a zwitterion ( ; ), also called an inner salt or dipolar ion, is a molecule that contains an equal number of positively and negatively charged functional groups. : (1,2- dipolar compounds, such as ylides, are sometimes excluded from the definition.) Some zwitterions, such as amino acid zwitterions, are in chemical equilibrium with an uncharged "parent" molecule. Betaines are zwitterions that cannot isomerize to an all-neutral form, such as when the positive charge is located on a quaternary ammonium group. Similarly, a molecule containing a phosphonium group and a carboxylate group cannot isomerize. Amino acids Tautomerism of amino acids follows this stoichiometry: : The ratio of the concentrations of the two species in solution is independent of pH. It has been suggested, on the basis of theoretical analysis, that the zwitterion is stabilized in aqueous solution by hydrogen bonding with solvent water molecules. Analysis of neutron diffraction data for g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. It is a common element in the universe, estimated at Abundance of the chemical elements, seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element chemical bond, bond to form N2, a colourless and odourless diatomic molecule, diatomic gas. N2 forms about 78% of Atmosphere of Earth, Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772 and independently by Carl Wilhelm Scheele and Henry Cavendish at about the same time. The name was suggested by French chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exocyclic
In organic chemistry, an alicyclic compound contains one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character. Alicyclic compounds may have one or more aliphatic side chains attached. Cycloalkanes The simplest alicyclic compounds are the monocyclic cycloalkanes: cyclopropane, cyclobutane, cyclopentane, cyclohexane, cycloheptane, cyclooctane, and so on. Bicyclic alkanes include decalin, housane, and norbornane. Polycyclic alkanes include cubane, basketane, and tetrahedrane. Spiro compounds have two or more rings that are connected through only one carbon atom. The mode of ring-closing in the formation of many alicyclic compounds can be predicted by Baldwin's rules. Otto Wallach, a German chemist, received the 1910 Nobel Prize in Chemistry for his work on alicyclic compounds. Cycloalkenes Monocyclic cycloalkenes are cyclopropene, cyclobutene, cyclopentene, cyclohexene, cycloheptene, cyclooctene, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stimulant
Stimulants (also known as central nervous system stimulants, or psychostimulants, or colloquially as uppers) are a class of drugs that increase alertness. They are used for various purposes, such as enhancing attention, motivation, cognition, Mood disorder, mood, and physical activity, physical performance. Some stimulants occur naturally, while others are exclusively synthetic. Common stimulants include caffeine, nicotine, amphetamines, cocaine, methylphenidate, and modafinil. Stimulants may be subject to varying forms of regulation, or outright prohibition, depending on jurisdiction. Stimulants increase activity in the sympathetic nervous system, either directly or indirectly. Prototypical stimulants increase synaptic concentrations of neurotransmitter, excitatory neurotransmitters, particularly norepinephrine and dopamine (e.g., methylphenidate). Other stimulants work by binding to the Receptor (biochemistry), receptors of excitatory neurotransmitters (e.g., nicotine) or by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |