|
Mercury(II) Cyanide
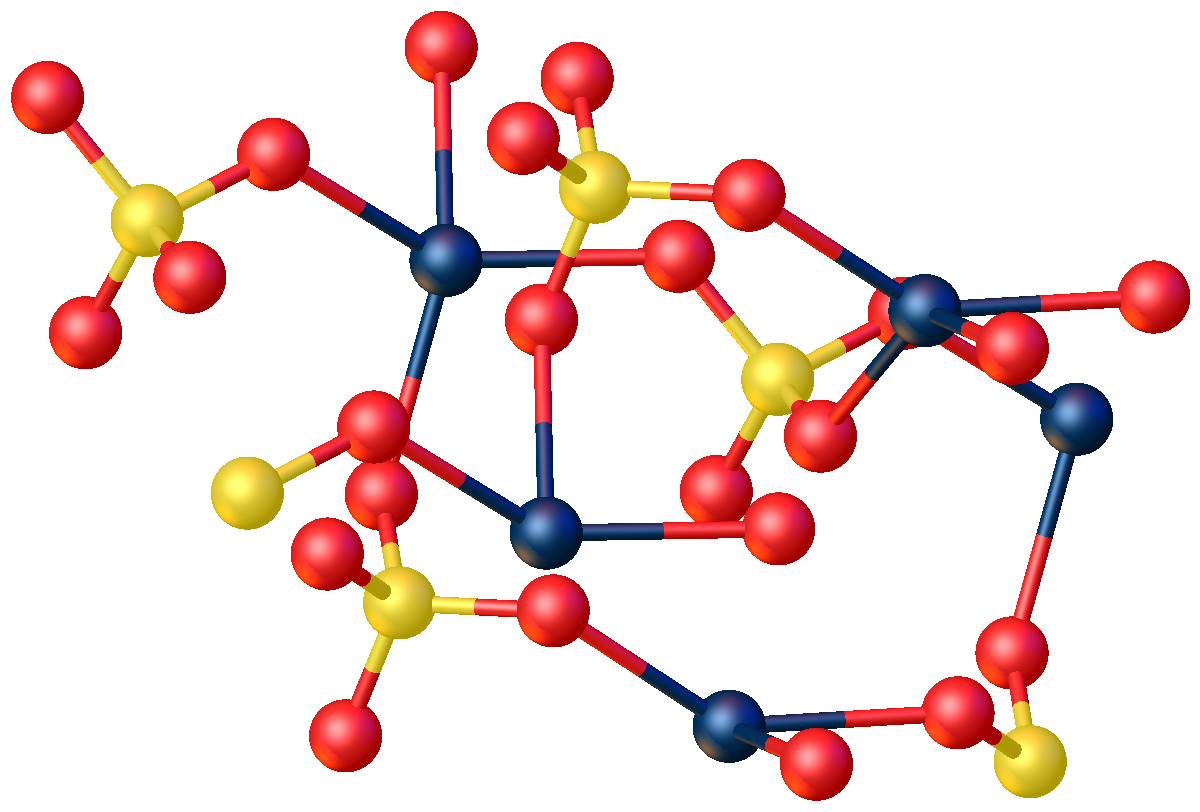
Mercury(II) cyanide, also known as mercuric cyanide, is a poisonous compound of mercury and cyanide. It is an odorless, toxic white powder. It is highly soluble in polar solvents such as water, alcohol, and ammonia, slightly soluble in ether, and insoluble in benzene and other hydrophobic solvents.Kocovsky, P., G. Wang, and V. Sharma. "Mercury(II) Cyanide." ''e-EROS Encyclopedia of Reagents for Organic Synthesis.'' Chichester, UK: John Wiley & Sons, Ltd., 2001. http://www.mrw.interscience.wiley.com/eros/articles/rm034/sect0-fs.html Molecular and crystal structure At ambient temperature and ambient pressure, Hg(CN)2 takes the form of tetragonal crystals. These crystals are composed of nearly linear Hg(CN)2 molecules with a C-Hg-C bond angle of 175.0° and an Hg-C-N bond angle of 177.0° (AylettAylett, B.J. "Mercury (II) Pseudohalides: Cyanide, Thiocyanate, Selenocyanate, Azide, Fulminate." ''Comprehensive Inorganic Chemistry'' 3:304-306. J.C. Bailar, Harry Julius Emeléus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the pseudoelement symbol for ethyl group, ethyl. Ethanol is a Volatility (chemistry), volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. Historically it was used as a general anesthetic, and has modern medical applications as an antiseptic, disinfectant, solvent for some medications, and antidote for methanol poisoning and ethylene glycol poisoning. It is used as a chemical solvent and in the Chemical synthesis, synthesis of orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (molecule), water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Ferrocyanide
Potassium hexacyanidoferrate(II) is the inorganic compound with formula K4 e(CN)6�3H2O. It is the potassium salt of the coordination complex e(CN)6sup>4−. This salt forms lemon-yellow monoclinic crystals. Synthesis In 1752, the French chemist Pierre Joseph Macquer (1718–1784) first reported the preparation of Potassium hexacyanidoferrate(II), which he achieved by reacting Prussian blue (iron(III) ferrocyanide) with potassium hydroxide. Modern production Potassium hexacyanidoferrate(II) is produced industrially from hydrogen cyanide, iron(II) chloride, and calcium hydroxide, the combination of which affords Ca2 e(CN)6�11H2O. This solution is then treated with potassium salts to precipitate the mixed calcium-potassium salt CaK2 e(CN)6 which in turn is treated with potassium carbonate to give the tetrapotassium salt. Historical production Historically, the compound was manufactured from nitrogenous organic material, iron filings, and potassium carbonate. Common nitrog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury(II) Sulfate
Mercury(II) sulfate, commonly called Mercury sulfate, mercuric sulfate, is the chemical compound Mercury (element), HgSulfur, SOxygen, O4. It is an odorless salt that forms white granules or crystalline powder. In water, it separates into an insoluble basic sulfate with a yellow color and sulfuric acid. Structure The anhydrous compound features Hg2+ in a highly distorted tetrahedral HgO4 environment. Two Hg-O distances are 2.22 Å and the others are 2.28 and 2.42 Å. In the monohydrate, Hg2+ adopts a linear coordination geometry with Hg-O (sulfate) and Hg-O (water) bond lengths of 2.179 and 2.228 Å, respectively. Four weaker bonds are also observed with Hg---O distances >2.5 Å. History In 1932, the Japanese chemical company Chisso Corporation began using mercury sulfate as the catalyst for the production of acetaldehyde from acetylene and water. Though it was unknown at the time, methylmercury is formed as a side product of this reaction. Exposure and consumption of the me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prussian Blue
Prussian blue (also known as Berlin blue, Brandenburg blue, Parisian and Paris blue) is a dark blue pigment produced by oxidation of ferrous ferrocyanide salts. It has the chemical formula . It consists of cations, where iron is in the oxidation state of +3, and anions, where iron is in the oxidation state of +2, so, the other name of this salt is iron(III) hexacyanoferrate(II). Turnbull's blue is essentially identical chemically, excepting that it has different impurities and particle sizes—because it is made from different reagents—and thus it has a slightly different color. Prussian blue was created in the early 18th century and is the first modern chemical synthesis, synthetic pigment. It is prepared as a very fine colloidal dispersion, because the compound is not soluble in water. It contains variable amounts of other ions and its appearance depends sensitively on the size of the colloidal particles. The pigment is used in paints, it became prominent in 19th-century ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aqueous Solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, also known as sodium chloride (NaCl), in water would be represented as . The word ''aqueous'' (which comes from ''aqua'') means pertaining to, related to, similar to, or dissolved in, water. As water is an excellent solvent and is also naturally abundant, it is a ubiquitous solvent in chemistry. Since water is frequently used as the solvent in experiments, the word solution refers to an aqueous solution, unless the solvent is specified. A ''non-aqueous solution'' is a solution in which the solvent is a liquid, but is not water. Characteristics Substances that are ''hydrophobic'' ('water-fearing') do not dissolve well in water, whereas those that are '' hydrophilic'' ('water-friendly') do. An example of a hydrophilic substance is sodium chloride. In an aqueous solution the hydrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis acids and bases, Lewis bases. The nature of metal–ligand bonding can range from covalent bond, covalent to ionic bond, ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acids and bases, Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity (chemistry), reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cubic Crystal System
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals. There are three main varieties of these crystals: *Primitive cubic (abbreviated ''cP'' and alternatively called simple cubic) *Body-centered cubic (abbreviated ''cI'' or bcc) *Face-centered cubic (abbreviated ''cF'' or fcc) Note: the term fcc is often used in synonym for the ''cubic close-packed'' or ccp structure occurring in metals. However, fcc stands for a face-centered cubic Bravais lattice, which is not necessarily close-packed when a motif is set onto the lattice points. E.g. the diamond and the zincblende lattices are fcc but not close-packed. Each is subdivided into other variants listed below. Although the ''unit cells'' in these crystals are conventionally taken to be cubes, the primitive unit cells often are not. Bravais lattices The three Bravais latices ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedral Symmetry
image:tetrahedron.svg, 150px, A regular tetrahedron, an example of a solid with full tetrahedral symmetry A regular tetrahedron has 12 rotational (or orientation-preserving) symmetries, and a symmetry order of 24 including transformations that combine a reflection and a rotation. The group of all (not necessarily orientation preserving) symmetries is isomorphic to the group S4, the symmetric group of permutations of four objects, since there is exactly one such symmetry for each permutation of the vertices of the tetrahedron. The set of orientation-preserving symmetries forms a group referred to as the alternating group, alternating subgroup A4 of S4. Details Chiral and full (or achiral tetrahedral symmetry and pyritohedral symmetry) are Point groups in three dimensions, discrete point symmetries (or equivalently, List of spherical symmetry groups, symmetries on the sphere). They are among the Crystal system#Overview of point groups by crystal system, crystallographic point gro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Complex (chemistry)
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing compounds, especially those that include transition metals (elements like titanium that belong to the periodic table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom through several of the ligand's atoms; ligands with 2, 3, 4 or even 6 bonds to the central a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bar (unit)
The bar is a metric unit of pressure defined as 100,000 Pa (100 kPa), though not part of the International System of Units (SI). A pressure of 1 bar is slightly less than the current average atmospheric pressure on Earth at sea level (approximately 1.013 bar). By the barometric formula, 1 bar is roughly the atmospheric pressure on Earth at an altitude of 111 metres at 15 °C. The bar and the millibar were introduced by the Norwegian meteorologist Vilhelm Bjerknes, who was a founder of the modern practice of weather forecasting, with the bar defined as one megadyne per square centimetre. The SI brochure, despite previously mentioning the bar, now omits any mention of it.. The bar has been legally recognised in countries of the European Union since 2004. British Standard BS 350:2004 ''Conversion Factors for Units''. The US National Institute of Standards and Technology (NIST) deprecates its use except for "limited use in meteorology" and lists ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |