|
Mandelate
Mandelic acid is an aromatic alpha hydroxy acid with the molecular formula C6H5CH(OH)CO2H. It is a white crystalline solid that is soluble in water and polar organic solvents. It is a useful precursor to various drugs. The molecule is chiral. The racemic mixture is known as ''paramandelic acid''. Isolation, synthesis, occurrence Mandelic acid was discovered in 1831 by the German pharmacist Ferdinand Ludwig Winckler (1801–1868) while heating amygdalin, an extract of bitter almonds, with diluted hydrochloric acid. The name is derived from the German "Mandel" for "almond". Mandelic acid is usually prepared by the acid-catalysed hydrolysis of mandelonitrile, which is the cyanohydrin of benzaldehyde. Mandelonitrile can also be prepared by reacting benzaldehyde with sodium bisulfite to give the corresponding adduct, forming mandelonitrile with sodium cyanide, which is then hydrolyzed: : Alternatively, it can be prepared by base hydrolysis of phenylchloroacetic acid as well as dibrom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
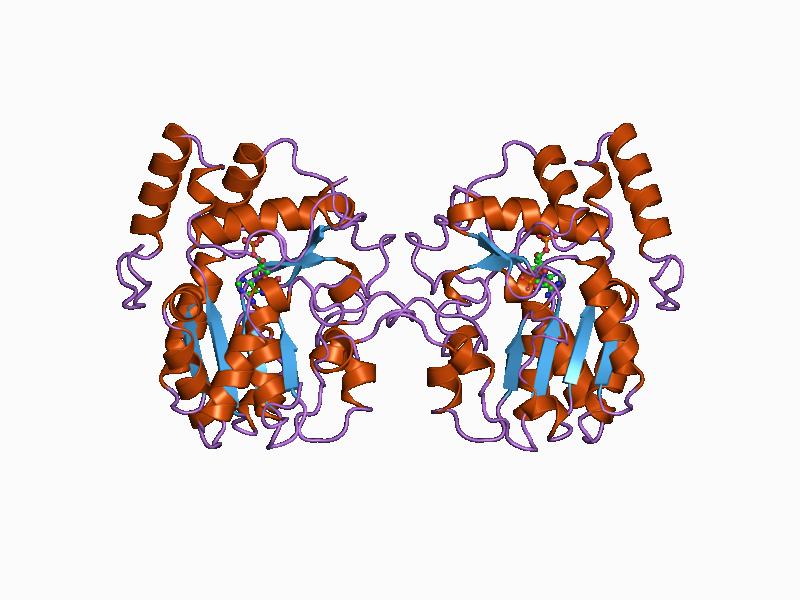
Mandelate Racemase
Mandelate racemase () is a bacterial enzyme which catalyzes the interconversion of the enantiomers of mandelate via an enol intermediate. This enzyme catalyses the following chemical reaction : (S)-mandelate \rightleftharpoons (R)-mandelate It is a member of the enolase superfamily of enzymes, along with muconate lactonizing enzyme and enolase Phosphopyruvate hydratase, usually known as enolase, is a metalloenzyme () that catalyses the conversion of 2-phosphoglycerate (2-PG) to phosphoenolpyruvate (PEP), the ninth and penultimate step of glycolysis. The chemical reaction is: :2-ph .... References External links * EC 5.1.2 {{Enzyme-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Merck Index
''The Merck Index'' is an encyclopedia of chemical substance, chemicals, pharmaceutical drug, drugs and biomolecule, biologicals with over 10,000 monographs on single substances or groups of related chemical compound, compounds published online by the Royal Society of Chemistry. History The first edition of the Merck's Index was published in 1889 by the German chemical company Merck Group, Emanuel Merck and was primarily used as a sales catalog for Merck's growing list of chemicals it sold. The American subsidiary was established two years later and continued to publish it. During World War I the US government seized Merck's US operations and made it a separate American "Merck" company that continued to publish the Merck Index. In 2012 the Merck Index was licensed to the Royal Society of Chemistry. An online version of The Merck Index, including historic records and new updates not in the print edition, is commonly available through research libraries. It also includes an append ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is among the simplest aromatic aldehydes and one of the most industrially useful. It is a colorless liquid with a characteristic almond-like odor, and is commonly used in cherry-flavored sodas. A component of bitter almond oil, benzaldehyde can be extracted from a number of other natural sources. Synthetic benzaldehyde is the flavoring agent in imitation almond extract, which is used to flavor cakes and other baked goods. History Benzaldehyde was first extracted in 1803 by the French pharmacist Martrès. His experiments focused on elucidating the nature of amygdalin, the poisonous compound found in bitter almonds, the fruit of '' Prunus dulcis''. Further work on the oil by Pierre Robiquet and Antoine Boutron Charlard, two French chemists, produced benzaldehyde. In 1832, Friedrich Wöhler and Justus von Liebig first synthesized benzaldehyde. Production Benzaldeh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transferase
In biochemistry, a transferase is any one of a class of enzymes that catalyse the transfer of specific functional groups (e.g. a methyl or glycosyl group) from one molecule (called the donor) to another (called the acceptor). They are involved in hundreds of different biochemical pathways throughout biology, and are integral to some of life's most important processes. Transferases are involved in myriad reactions in the cell. Three examples of these reactions are the activity of coenzyme A (CoA) transferase, which transfers thiol esters, the action of N-acetyltransferase, which is part of the pathway that metabolizes tryptophan, and the regulation of pyruvate dehydrogenase (PDH), which converts pyruvate to acetyl CoA. Transferases are also utilized during translation. In this case, an amino acid chain is the functional group transferred by a peptidyl transferase. The transfer involves the removal of the growing amino acid chain from the tRNA molecule in the A-site of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoamine Oxidase
Monoamine oxidases (MAO) () are a family of enzymes that catalyze the oxidation of monoamines, employing oxygen to clip off their amine group. They are found bound to the outer membrane of mitochondria in most cell types of the body. The first such enzyme was discovered in 1928 by Mary Bernheim in the liver and was named tyramine oxidase. The MAOs belong to the protein family of flavin-containing amine oxidoreductases. MAOs are important in the breakdown of monoamines ingested in food, and also serve to inactivate monoamine neurotransmitters. Because of the latter, they are involved in a number of psychiatric and neurological diseases, some of which can be treated with monoamine oxidase inhibitors (MAOIs) which block the action of MAOs. Subtypes and tissue distribution In humans there are two types of MAO: MAO-A and MAO-B. * Both are found in neurons and astroglia. * Outside the central nervous system: ** MAO-A is also found in the liver, pulmonary vascular end ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Noradrenaline
Norepinephrine (NE), also called noradrenaline (NA) or noradrenalin, is an organic chemical in the catecholamine family that functions in the brain and body as a hormone, neurotransmitter and neuromodulator. The name "noradrenaline" (from Latin '' ad'', "near", and '' ren'', "kidney") is more commonly used in the United Kingdom and the rest of the world, whereas "norepinephrine" (from Ancient Greek ἐπῐ́ (''epí''), "upon", and νεφρός (''nephrós''), "kidney") is usually preferred in the United States. "Norepinephrine" is also the international nonproprietary name given to the drug. Regardless of which name is used for the substance itself, parts of the body that produce or are affected by it are referred to as noradrenergic. The general function of norepinephrine is to mobilize the brain and body for action. Norepinephrine release is lowest during sleep, rises during wakefulness, and reaches much higher levels during situations of stress or danger, in the so ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adrenaline
Adrenaline, also known as epinephrine, is a hormone and medication which is involved in regulating visceral functions (e.g., respiration). It appears as a white microcrystalline granule. Adrenaline is normally produced by the adrenal glands and by a small number of neurons in the medulla oblongata. It plays an essential role in the fight-or-flight response by increasing blood flow to muscles, heart output by acting on the SA node, pupil dilation response, and blood sugar level. It does this by binding to alpha and beta receptors. It is found in many animals, including humans, and some single-celled organisms. It has also been isolated from the plant '' Scoparia dulcis'' found in Northern Vietnam. Medical uses As a medication, it is used to treat several conditions, including allergic reaction anaphylaxis, cardiac arrest, and superficial bleeding. Inhaled adrenaline may be used to improve the symptoms of croup. It may also be used for asthma when other treatments a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylpyruvic Acid
Phenylpyruvic acid is the organic compound with the formula C6H5CH2C(O)CO2H. It is a keto acid. Occurrence and properties The compound exists in equilibrium with its (''E'')- and (''Z'')-enol tautomers. It is a product from the oxidative deamination of phenylalanine. When the activity of the enzyme phenylalanine hydroxylase is reduced, the amino acid phenylalanine accumulates and gets converted into phenylpyruvic acid (phenylpyruvate), which leads to ' Phenylketonuria (PKU)' instead of 'tyrosine' which is the normal product of phenylalanine hydroxylase. Preparation and reactions It can be prepared by many methods. Classically it is produced from aminocinnamic acid derivatives. It has been prepared by condensation of benzaldehyde and glycine derivatives to give phenylazlactone, which is then hydrolyzed with acid- or base-catalysis. It can also be synthesized from benzyl chloride by double carbonylation. Reductive amination of phenylpyruvic acid gives phenylalanine. See also * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxylation
In chemistry, hydroxylation refers to the installation of a hydroxyl group () into an organic compound. Hydroxylations generate alcohols and phenols, which are very common functional groups. Hydroxylation confers some degree of water-solubility. Hydroxylation of a hydrocarbon is an oxidation, thus a step in degradation. Biological hydroxylation In biochemistry, hydroxylation reactions are often facilitated by enzymes called hydroxylases. These enzymes insert an O atom into a bond. Typical stoichiometries for the hydroxylation of a generic hydrocarbon are these: : : Since itself is a slow and unselective hydroxylating agent, catalysts are required to accelerate the pace of the process and to introduce selectivity. Hydroxylation is often the first step in the degradation of organic compounds in air. Hydroxylation is important in detoxification since it converts lipophilic compounds into water-soluble (hydrophilic) products that are more readily removed by the kidneys or liver ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cinnamic Acid
Cinnamic acid is an organic compound with the formula phenyl, C6H5-CH=CH-Carboxylic acid, COOH. It is a white crystalline compound that is slightly soluble in water, and freely soluble in many organic solvents. Classified as an unsaturated carboxylic acid, it occurs naturally in a number of plants. It exists as both a Cis–trans isomerism, ''cis'' and a ''trans'' isomer, although the latter is more common. The ''cis''-isomer is called allocinnamic acid. Occurrence and production Biosynthesis Cinnamic acid is a central intermediate in the biosynthesis of a myriad of natural products including lignols (precursors to lignin and lignocellulose), flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids. Its biosynthesis involves the action of the enzyme phenylalanine ammonia-lyase (PAL) on phenylalanine. Natural occurrence It is obtained from oil of cinnamon, or from balsams such as storax. It is also found in shea butter. Cinnamic acid has a honey-like ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylglyoxal
Phenylglyoxal is the organic compound with the formula C6H5C(O)C(O)H. It contains both an aldehyde and a ketone functional group. It is yellow liquid when anhydrous but readily forms a colorless crystalline hydrate. It has been used as a reagent to modify the amino acid, arginine. It has also been used to attach chemical payload (probes) to the amino acid citrulline and to peptides/proteins. Properties Like some other aldehydes, phenylglyoxal polymerizes upon standing, as indicated by solidification of the liquid. Upon heating, this polymer "cracks" to give back the yellow aldehyde. Dissolution of phenylglyoxal in water gives crystals of the hydrate: : C6H5C(O)COH + H2O → C6H5C(O)CH(OH)2 Upon heating, the hydrate loses water and regenerates the anhydrous liquid. Preparation Phenylglyoxal was first prepared by thermal decomposition of the sulfite derivative of the oxime: : C6H5C(O)CH(NOSO2H) + 2 H2O → C6H5C(O)CHO + NH4HSO4 It may also be prepared from methyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Preparation Of Mandelic Acid
Preparation may refer to: * Preparation (dental), the method by which a tooth is prepared when removing decay and designing a form that will provide adequate retention for a dental restoration * Preparation (music), treatment of dissonance in tonal music * Preparation, Iowa, a ghost town * Preparation time, time to prepare speeches in policy debate * '' The Preparation'', a 2017 South Korean film * ''Preparations'' (album), a 2007 album by Prefuse 73 * Prepared dosage form * Prepared drug * Prepared food * Prepared supplement * Special modifications to instruments, see **Prepared piano **Prepared guitar *Fossil preparation Fossil preparation is a complex of tasks that can include excavating, revealing, conserving, and replicating the ancient remains and traces of organisms. It is an integral part of the science of paleontology, of museum exhibition, and the preser ... See also * Preparation H, popular hemorrhoids medicine * Preparation for the Gospel, early Christian book ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |