|
Lithium Monoxide Anion
Lithium monoxide anion (LiO−) is a superbase existing in the gas phase. It was the strongest known base until 2008, when the isomeric diethynylbenzene dianions were determined to have a higher proton affinity. The methanide ion CH3− was the strongest known base before lithium monoxide anion was discovered. LiO− has a proton affinity The proton affinity (PA, ''E''pa) of an anion or of a neutral atom or molecule is the negative of the enthalpy change in the reaction between the chemical species concerned and a proton in the gas phase: ::: A- + H+ -> HA ::: B + H+ -> BH ... of ~1782 kJ mol−1. Synthesis of the lithium monoxide anion The anion is prepared in a mass spectrometer by successive decarboxylation and decarbonylation of lithium oxalate anion under collision induced dissociation (CID) conditions: LiO(C=O)(CO) → LiOCO− + CO2, LiOCO− → LiO− + CO. The above method to synthesize the lithium monoxide anion is inefficient and difficult to carry ou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Hydroxide
Lithium hydroxide is an inorganic compound with the formula LiOH. It can exist as anhydrous or hydrated, and both forms are white hygroscopic solids. They are soluble in water and slightly soluble in ethanol. Both are available commercially. While classified as a strong base, lithium hydroxide is the weakest known alkali metal hydroxide. Production The preferred feedstock is hard-rock spodumene, where the lithium content is expressed as % lithium oxide. Lithium carbonate route Lithium hydroxide is often produced industrially from lithium carbonate in a metathesis reaction with calcium hydroxide: : The initially produced hydrate is dehydrated by heating under vacuum up to 180 °C. Lithium sulfate route An alternative route involves the intermediacy of lithium sulfate: :α-spodumene → β-spodumene :β-spodumene + CaO → + ... : : The main by-products are gypsum and sodium sulphate, which have some market value. Commercial setting According to Bloomberg, Ganfeng Lit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Base (chemistry)
In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century. In 1884, Svante Arrhenius proposed that a base is a substance which dissociates in aqueous solution to form Hydroxide ions OH−. These ions can react with hydrogen ions (H+ according to Arrhenius) from the dissociation of acids to form water in an acid–base reaction. A base was therefore a metal hydroxide such as NaOH or Ca(OH)2. Such aqueous hydroxide solutions were also described by certain characteristic properties. They are slippery to the touch, can taste bitter and change the color of pH indicators (e.g., turn red litmus paper blue). In water, by altering the autoionization equilibrium, bases yield solutions in which the hydrogen ion activity is lower than it is in pure water, i.e., the water ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Meta-diethynylbenzene Dianion
In organic chemistry, a diethynylbenzene dianion is an anion consisting of two ethynyl anions as substituents on a benzene ring. With the chemical formula , three positional isomers are possible, differing in the relative positions of the two substituents around the ring: *''ortho''-diethynylbenzene dianion *''meta''-diethynylbenzene dianion *''para''-diethynylbenzene dianion The gaseous state of all three anions are of theoretical interest. They have been generated by decarboxylation of benzene di propynoic acids, using the technique of mass spectrometry. The three isomers of the dianion are the three strongest known superbases ever, with the ''ortho'' isomer being the strongest, with a proton affinity of . The ''meta'' isomer is the second-strongest, and the ''para'' isomer is the third-strongest. Observation These dianions were generated in a linear quadrupole ion-trap mass spectrometer. Electrospray ionization (ESI) of the diacid precursor results in the dicarboxylate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Para-diethynylbenzene Dianion
In organic chemistry, a diethynylbenzene dianion is an anion consisting of two ethynyl anions as substituents on a benzene ring. With the chemical formula , three positional isomers are possible, differing in the relative positions of the two substituents around the ring: *''ortho''-diethynylbenzene dianion *''meta''-diethynylbenzene dianion *''para''-diethynylbenzene dianion The gaseous state of all three anions are of theoretical interest. They have been generated by decarboxylation of benzene di propynoic acids, using the technique of mass spectrometry. The three isomers of the dianion are the three strongest known superbases ever, with the ''ortho'' isomer being the strongest, with a proton affinity of . The ''meta'' isomer is the second-strongest, and the ''para'' isomer is the third-strongest. Observation These dianions were generated in a linear quadrupole ion-trap mass spectrometer. Electrospray ionization (ESI) of the diacid precursor results in the dicarboxylate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ortho-diethynylbenzene Dianion
In organic chemistry, a diethynylbenzene dianion is an anion consisting of two ethynyl anions as substituents on a benzene ring. With the chemical formula , three positional isomers are possible, differing in the relative positions of the two substituents around the ring: *''ortho''-diethynylbenzene dianion *''meta''-diethynylbenzene dianion *''para''-diethynylbenzene dianion The gaseous state of all three anions are of theoretical interest. They have been generated by decarboxylation of benzene di propynoic acids, using the technique of mass spectrometry. The three isomers of the dianion are the three strongest known superbases ever, with the ''ortho'' isomer being the strongest, with a proton affinity of . The ''meta'' isomer is the second-strongest, and the ''para'' isomer is the third-strongest. Observation These dianions were generated in a linear quadrupole ion-trap mass spectrometer. Electrospray ionization (ESI) of the diacid precursor results in the dicarboxylate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
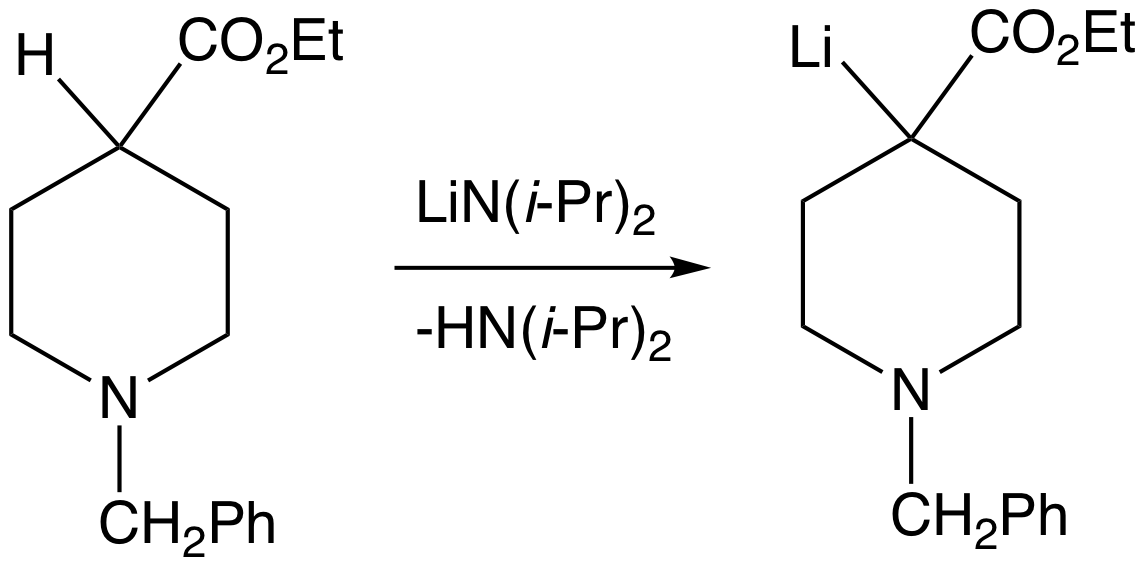
Superbase
A superbase is a compound that has a particularly high affinity for protons. Superbases are of theoretical interest and potentially valuable in organic synthesis. Superbases have been described and used since the 1850s.''Superbases for Organic Synthesis'' Ed. Ishikawa, T., John Wiley and Sons, Ltd.: West Sussex, UK. 2009. Definitions Generically IUPAC defines a superbase as a "compound having a very high basicity, such as lithium diisopropylamide." Superbases are often defined in two broad categories, organic and organometallic. Organic superbases are charge-neutral compounds with basicities greater than that of proton sponge (pKBH+ = 18.6 in MeCN)." In a related definition: any species with a higher absolute proton affinity (APA = 245.3 kcal/mol) and intrinsic gas phase basicity (GB = 239 kcal/mol) than proton sponge. Common superbases of this variety feature amidine, guanidine, and phosphazene functional groups. Strong superbases can be designed by utilizing multiple int ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ortho-Diethynylbenzene Dianion
In organic chemistry, a diethynylbenzene dianion is an anion consisting of two ethynyl anions as substituents on a benzene ring. With the chemical formula , three positional isomers are possible, differing in the relative positions of the two substituents around the ring: *''ortho''-diethynylbenzene dianion *''meta''-diethynylbenzene dianion *''para''-diethynylbenzene dianion The gaseous state of all three anions are of theoretical interest. They have been generated by decarboxylation of benzene di propynoic acids, using the technique of mass spectrometry. The three isomers of the dianion are the three strongest known superbases ever, with the ''ortho'' isomer being the strongest, with a proton affinity of . The ''meta'' isomer is the second-strongest, and the ''para'' isomer is the third-strongest. Observation These dianions were generated in a linear quadrupole ion-trap mass spectrometer. Electrospray ionization (ESI) of the diacid precursor results in the dicarboxylate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Anion
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bounded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For example, protonation of methanol gives an elec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proton Affinity
The proton affinity (PA, ''E''pa) of an anion or of a neutral atom or molecule is the negative of the enthalpy change in the reaction between the chemical species concerned and a proton in the gas phase: ::: A- + H+ -> HA ::: B + H+ -> BH+ These reactions are always exothermic in the gas phase, i.e. energy is released (enthalpy is negative) when the reaction advances in the direction shown above, while the proton affinity is positive. This is the same sign convention used for electron affinity. The property related to the proton affinity is the gas-phase basicity, which is the negative of the Gibbs energy for above reactions, i.e. the gas-phase basicity includes entropic terms in contrast to the proton affinity. Acid/base chemistry The higher the proton affinity, the stronger the base and the weaker the conjugate acid ''in the gas phase''. The (reportedly) strongest known base is the ortho-diethynylbenzene dianion (''E''pa = 1843 kJ/mol), followed by the methan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mole (unit)
The mole, symbol mol, is the unit of amount of substance in the International System of Units (SI). The quantity amount of substance is a measure of how many elementary entities of a given substance are in an object or sample. The mole is defined as containing exactly elementary entities. Depending on what the substance is, an elementary entity may be an atom, a molecule, an ion, an ion pair, or a subatomic particle such as an electron. For example, 10 moles of water (a chemical compound) and 10 moles of mercury (a chemical element), contain equal amounts of substance and the mercury contains exactly one atom for each molecule of the water, despite the two having different volumes and different masses. The number of elementary entities in one mole is known as the Avogadro number, which is the approximate number of nucleons (protons or neutrons) in one gram of ordinary matter. The previous definition of a mole was simply the number of elementary entities equal to that of 12 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Oxide
Lithium oxide ( O) or lithia is an inorganic chemical compound. It is a white solid. Although not specifically important, many materials are assessed on the basis of their Li2O content. For example, the Li2O content of the principal lithium mineral spodumene (LiAlSi2O6) is 8.03%. Production Lithium oxide is produced by thermal decomposition of lithium peroxide at 300–400 °C.Wietelmann, Ulrich and Bauer, Richard J. (2005) "Lithium and Lithium Compounds" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH: Weinheim. . Lithium oxide forms along with small amounts of lithium peroxide when lithium metal is burned in the air at and combines with oxygen at temperatures above 100 °C: :4Li + → 2. Pure can be produced by the thermal decomposition of lithium peroxide, , at 450 °C :2 → 2 + Structure Solid lithium oxide adopts an antifluorite structure with four-coordinated Li+ centers and eight-coordinated oxides. The ground state gas phase molec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superbases
A superbase is a compound that has a particularly high affinity for protons. Superbases are of theoretical interest and potentially valuable in organic synthesis. Superbases have been described and used since the 1850s.''Superbases for Organic Synthesis'' Ed. Ishikawa, T., John Wiley and Sons, Ltd.: West Sussex, UK. 2009. Definitions Generically IUPAC defines a superbase as a "compound having a very high basicity, such as lithium diisopropylamide." Superbases are often defined in two broad categories, organic and organometallic. Organic superbases are charge-neutral compounds with basicities greater than that of proton sponge (pKBH+ = 18.6 in MeCN)." In a related definition: any species with a higher absolute proton affinity (APA = 245.3 kcal/mol) and intrinsic gas phase basicity (GB = 239 kcal/mol) than proton sponge. Common superbases of this variety feature amidine, guanidine, and phosphazene functional groups. Strong superbases can be designed by utilizing multiple intra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |