|
Lithium Iodide
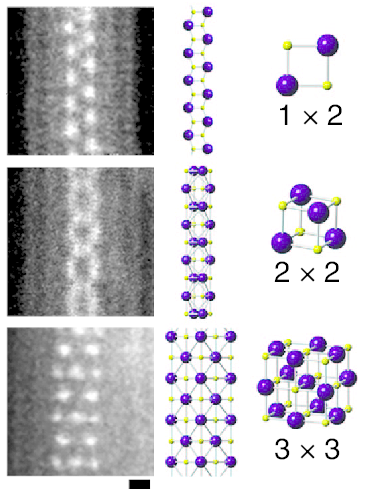
Lithium iodide, or LiI, is a compound of lithium and iodine. When exposed to air, it becomes yellow in color, due to the oxidation of iodide to iodine. It crystallizes in the NaCl motif. It can participate in various hydrates.Wietelmann, Ulrich and Bauer, Richard J. (2005) "Lithium and Lithium Compounds" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH: Weinheim. . Applications Lithium iodide is used as a solid-state electrolyte for high-temperature batteries. It is also the standard electrolyte in artificial pacemakers due to the long cycle life it enables. The solid is used as a phosphor for neutron detection. It is also used, in a complex with Iodine, in the electrolyte of dye-sensitized solar cells. In organic synthesis, LiI is useful for cleaving C-O bonds. For example, it can be used to convert methyl esters to carboxylic acids: :RCO2CH3 + LiI → RCO2Li + CH3I Similar reactions apply to epoxides and aziridines. Lithium iodide was used as a radiocontrast ag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid element. Like all alkali metals, lithium is highly reactive and flammable, and must be stored in vacuum, inert atmosphere, or inert liquid such as purified kerosene or mineral oil. When cut, it exhibits a metallic luster, but moist air corrodes it quickly to a dull silvery gray, then black tarnish. It never occurs freely in nature, but only in (usually ionic) compounds, such as pegmatitic minerals, which were once the main source of lithium. Due to its solubility as an ion, it is present in ocean water and is commonly obtained from brines. Lithium metal is isolated electrolytically from a mixture of lithium chloride and potassium chloride. The nucleus of the lithium atom verges on instability, since the two stable lithium isotopes foun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a violet gas at . The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek 'violet-coloured'. Iodine occurs in many oxidation states, including iodide (I−), iodate (), and the various periodate anions. It is the least abundant of the stable halogens, being the sixty-first most abundant element. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. The dominant producers of iodine today are Chile and Japan. Due to its high atomic number and ease of attachment to organic compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epoxide
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale for many applications. In general, low molecular weight epoxides are colourless and nonpolar, and often volatile. Nomenclature A compound containing the epoxide functional group can be called an epoxy, epoxide, oxirane, and ethoxyline. Simple epoxides are often referred to as oxides. Thus, the epoxide of ethylene (C2H4) is ethylene oxide (C2H4O). Many compounds have trivial names; for instance, ethylene oxide is called "oxirane". Some names emphasize the presence of the epoxide functional group, as in the compound ''1,2-epoxyheptane'', which can also be called ''1,2-heptene oxide''. A polymer formed from epoxide precursors is called an ''epoxy'', but such materials do not contain epoxide groups (or contain only a few residual epoxy grou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They at oftentimes have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid (C3H7CO2H) is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on another ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: ''total synthesis'', ''semisynthesis'', and ''methodology''. Total synthesis A total synthesis is the complete chemical synthesis of complex organic molecules from simple, commercially available petrochemical or natural precursors. Total synthesis may be accomplished either via a linear or convergent approach. In a ''linear'' synthesis—often adequate for simple structures—several steps are performed one after another until the molecule is complete; the chemical compounds made in each step are called synthetic intermediates. Most often, each step in a synthesis refers to a separate rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dye-sensitized Solar Cell
A dye-sensitized solar cell (DSSC, DSC, DYSC or Grätzel cell) is a low-cost solar cell belonging to the group of thin film solar cells. It is based on a semiconductor formed between a photo-sensitized anode and an electrolyte, a '' photoelectrochemical'' system. The modern version of a dye solar cell, also known as the Grätzel cell, was originally co-invented in 1988 by Brian O'Regan and Michael Grätzel at UC Berkeley and this work was later developed by the aforementioned scientists at the École Polytechnique Fédérale de Lausanne (EPFL) until the publication of the first high efficiency DSSC in 1991. Michael Grätzel has been awarded the 2010 Millennium Technology Prize for this invention. The DSSC has a number of attractive features; it is simple to make using conventional roll-printing techniques, is semi-flexible and semi-transparent which offers a variety of uses not applicable to glass-based systems, and most of the materials used are low-cost. In practice it has p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolyte
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved. Electrically, such a solution is neutral. If an electric potential is applied to such a solution, the cations of the solution are drawn to the electrode that has an abundance of electrons, while the anions are drawn to the electrode that has a deficit of electrons. The movement of anions and cations in opposite directions within the solution amounts to a current. Some gases, such as hydrogen chloride (HCl), under conditions of high temperature or low pressure can also function as electrolytes. El ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. Their properties and interactions are described by nuclear physics. Protons and neutrons are not elementary particles; each is composed of three quarks. The chemical properties of an atom are mostly determined by the configuration of electrons that orbit the atom's heavy nucleus. The electron configuration is determined by the charge of the nucleus, which is determined by the number of protons, or atomic number. The number of neutrons is the neutron number. Neutrons do not affect the electron configuration, but the sum of atomic and neutron numbers is the mass of the nucleus. Atoms of a chemical element t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphor
A phosphor is a substance that exhibits the phenomenon of luminescence; it emits light when exposed to some type of radiant energy. The term is used both for fluorescent or phosphorescent substances which glow on exposure to ultraviolet or visible light, and cathodoluminescent substances which glow when struck by an electron beam (cathode rays) in a cathode-ray tube. When a phosphor is exposed to radiation, the orbital electrons in its molecules are excited to a higher energy level; when they return to their former level they emit the energy as light of a certain color. Phosphors can be classified into two categories: fluorescent substances which emit the energy immediately and stop glowing when the exciting radiation is turned off, and phosphorescent substances which emit the energy after a delay, so they keep glowing after the radiation is turned off, decaying in brightness over a period of milliseconds to days. Fluorescent materials are used in applications in which the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Artificial Pacemaker
An artificial cardiac pacemaker (or artificial pacemaker, so as not to be confused with the natural cardiac pacemaker) or pacemaker is a medical device that generates electrical impulses delivered by electrodes to the chambers of the heart either the upper atria, or lower ventricles to cause the targeted chambers to contract and pump blood. By doing so, the pacemaker regulates the function of the electrical conduction system of the heart. The primary purpose of a pacemaker is to maintain an adequate heart rate, either because the heart's natural pacemaker is not fast enough, or because there is a block in the heart's electrical conduction system. Modern pacemakers are externally programmable and allow a cardiologist, particularly a cardiac electrophysiologist, to select the optimal pacing modes for individual patients. Most pacemakers are on demand, in which the stimulation of the heart is based on the dynamic demand of the circulatory system. Others send out a fixed rate of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
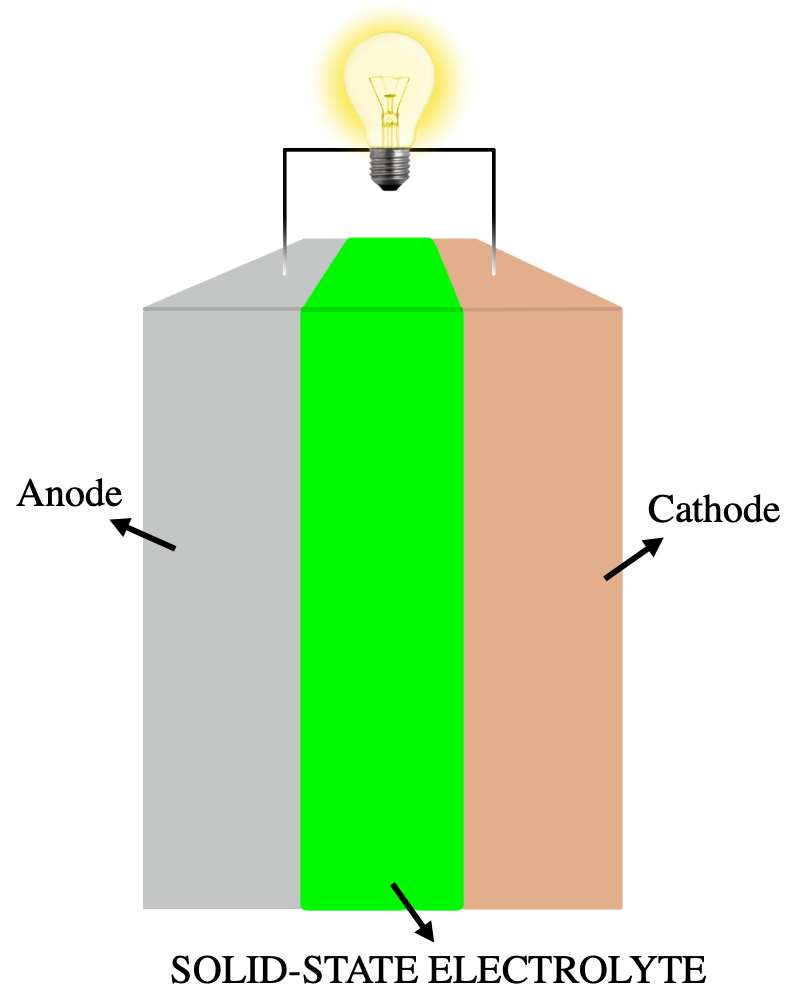
Solid-state Electrolyte
A solid-state electrolyte (SSE) is a solid Ionic conductivity (solid state), ionic conductor and electron-insulating electrolyte, material and it is the characteristic component of the solid-state battery. It is useful for applications in electrical energy storage (EES) in substitution of the liquid electrolytes found in particular in lithium-ion battery. The main advantages are the absolute safety, no issues of leakages of toxic organic liquid, organic solvents, low flammability, non-volatility, mechanical and thermal stability, easy processability, low self-discharge, higher achievable power density and cyclability. This makes possible, for example, the use of a lithium metal anode in a practical device, without the intrinsic limitations of a liquid electrolyte thanks to the property of lithium dendrite suppression in the presence of a solid-state electrolyte membrane. The utilization of a high capacity anode and low reduction potential, like lithium with a specific capacity of 38 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |