|
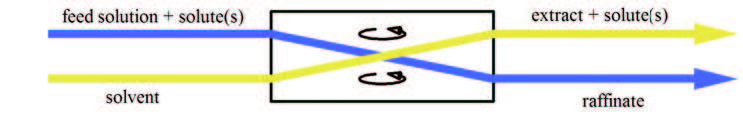
Liquid–liquid Extraction
Liquid–liquid extraction (LLE), also known as solvent extraction and partitioning, is a method to separate compounds or metal complexes, based on their relative solubilities in two different immiscible liquids, usually water (polar) and an organic solvent (non-polar). There is a net transfer of one or more species from one liquid into another liquid phase, generally from aqueous to organic. The transfer is driven by chemical potential, i.e. once the transfer is complete, the overall system of chemical components that make up the solutes and the solvents are in a more stable configuration (lower free energy). The solvent that is enriched in solute(s) is called extract. The feed solution that is depleted in solute(s) is called the raffinate. LLE is a basic technique in chemical laboratories, where it is performed using a variety of apparatus, from separatory funnels to countercurrent distribution equipment called as mixer settlers. This type of process is commonly performed after ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solvent Extraction
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell. The quantity of solute that can dissolve in a specific volume of solvent varies with temperature. Major uses of solvents are in paints, paint removers, inks, and dry cleaning. Specific uses for organic solvents are in dry cleaning (e.g. tetrachloroethylene); as paint thinners (toluene, turpentine); as nail polish removers and solvents of glue (acetone, methyl acetate, ethyl acetate); in spot removers (hexane, petrol ether); in detergents ( citrus terpenes); and in perfumes (ethanol). Solvents find various applications in chemical, pharmaceutical, oil, and gas industries, including in chemical s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). Electrodes are essential parts of batteries that can consist of a variety of materials depending on the type of battery. The electrophore, invented by Johan Wilcke, was an early version of an electrode used to study static electricity. Anode and cathode in electrochemical cells Electrodes are an essential part of any battery. The first electrochemical battery made was devised by Alessandro Volta and was aptly named the Voltaic cell. This battery consisted of a stack of copper and zinc electrodes separated by brine-soaked paper disks. Due to fluctuation in the voltage provided by the voltaic cell it wasn't very practical. The first practical battery was invented in 1839 and named the Daniell cell after John Frederic Daniell. Still making use of the zinc–copper electrode combination. Since then many more batteries ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Partition Coefficient
In the physical sciences, a partition coefficient (''P'') or distribution coefficient (''D'') is the ratio of concentrations of a compound in a mixture of two immiscible solvents at equilibrium. This ratio is therefore a comparison of the solubilities of the solute in these two liquids. The partition coefficient generally refers to the concentration ratio of un-ionized species of compound, whereas the distribution coefficient refers to the concentration ratio of all species of the compound (ionized plus un-ionized). In the chemical and pharmaceutical sciences, both phases usually are solvents. Most commonly, one of the solvents is water, while the second is hydrophobic, such as 1-octanol. Hence the partition coefficient measures how hydrophilic ("water-loving") or hydrophobic ("water-fearing") a chemical substance is. Partition coefficients are useful in estimating the distribution of drugs within the body. Hydrophobic drugs with high octanol-water partition coefficients are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aqueous Phase
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would be represented as . The word ''aqueous'' (which comes from ''aqua'') means pertaining to, related to, similar to, or dissolved in, water. As water is an excellent solvent and is also naturally abundant, it is a ubiquitous solvent in chemistry. Since water is frequently used as the solvent in experiments, the word solution refers to an aqueous solution, unless the solvent is specified. A ''non-aqueous solution'' is a solution in which the solvent is a liquid, but is not water. (See also Solvent and Inorganic nonaqueous solvent.) Characteristics Substances that are ''hydrophobic'' ('water-fearing') do not dissolve well in water, whereas those that are ''hydrophilic'' ('water-friendly') do. An example of a hydrophilic substance is sodium chlo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solute
In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. If the attractive forces between the solvent and solute particles are greater than the attractive forces holding the solute particles together, the solvent particles pull the solute particles apart and surround them. These surrounded solute particles then move away from the solid solute and out into the solution. The mixing process of a solution happens at a scale where the effects of chemical polarity are involved, resulting in interactions that are specific to solvation. The solution usually has the state of the solvent when the solvent is the larger fraction of the mixture, as is commonly the case. One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term "aqueous solution" is used when ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', ''molar concentration'', '' number concentration'', and '' volume concentration''. The concentration can refer to any kind of chemical mixture, but most frequently refers to solutes and solvents in solutions. The molar (amount) concentration has variants, such as normal concentration and osmotic concentration. Etymology The term concentration comes from the word concentrate, from the French , from con– + center, meaning “to put at the center”. Qualitative description Often in informal, non-technical language, concentration is described in a qualitative way, through the use of adjectives such as "dilute" for solutions of relatively low concentration and "concentrated" for solutions of relatively high concentration. To concentrate a solution, one must add more solute (for exam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Third Phase
Third phase is the term for a stable emulsion which forms in a liquid–liquid extraction when the original two phases (aqueous and organic) are mixed. The third phase can be caused by a detergent (surfactant) or a fine solid. While third phase is a term for an unwanted emulsion, a stable emulsion is wanted in emulsion polymerization all the things which can be used to make a stable 'emulsion' for a latex synthesis can prove to encourage a third phase to form. One term for the third phase found in PUREX plants is crud (Chalk River Unknown Deposit). One common crud is formed by the reaction of zirconium salts (from fission) with degraded tributyl phosphate (TBP). The TBP degrades into dibutyl hydrogen phosphate and then into butyl dihydrogen phosphate. The dibutyl hydrogen phosphate and the zirconium can form polymer A polymer (; Greek '' poly-'', "many" + '' -mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Emulsion
An emulsion is a mixture of two or more liquids that are normally immiscible (unmixable or unblendable) owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloids. Although the terms ''colloid'' and ''emulsion'' are sometimes used interchangeably, ''emulsion'' should be used when both phases, dispersed and continuous, are liquids. In an emulsion, one liquid (the dispersed phase) is dispersed in the other (the continuous phase). Examples of emulsions include vinaigrettes, homogenized milk, liquid biomolecular condensates, and some cutting fluids for metal working. Two liquids can form different types of emulsions. As an example, oil and water can form, first, an oil-in-water emulsion, in which the oil is the dispersed phase, and water is the continuous phase. Second, they can form a water-in-oil emulsion, in which water is the dispersed phase and oil is the continuous phase. Multiple emulsions are also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid
Solid is one of the four fundamental states of matter (the others being liquid, gas, and plasma). The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structural rigidity and resistance to a force applied to the surface. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire available volume like a gas. The atoms in a solid are bound to each other, either in a regular geometric lattice ( crystalline solids, which include metals and ordinary ice), or irregularly (an amorphous solid such as common window glass). Solids cannot be compressed with little pressure whereas gases can be compressed with little pressure because the molecules in a gas are loosely packed. The branch of physics that deals with solids is called solid-state physics, and is the main branch of condensed matter physics (which also includes liquids). Materials s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Detergent
A detergent is a surfactant or a mixture of surfactants with cleansing properties when in dilute solutions. There are a large variety of detergents, a common family being the alkylbenzene sulfonates, which are soap-like compounds that are more soluble in hard water, because the polar sulfonate (of detergents) is less likely than the polar carboxylate (of soap) to bind to calcium and other ions found in hard water. Definitions The word ''detergent'' is derived from the Latin adjective ''detergens'', from the verb ''detergere'', meaning to wipe or polish off. Detergent is a surfactant or a mixture of surfactants with cleansing properties when in dilute solutions. However, conventionally, detergent is used to mean synthetic cleaning compounds as opposed to ''soap'' (a salt of the natural fatty acid), even though soap is also a detergent in the true sense. In domestic contexts, the term ''detergent'' refers to household cleaning products such as '' laundry detergent'' o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Amalgam
Sodium amalgam, commonly denoted Na(Hg), is an alloy of mercury and sodium. The term amalgam is used for alloys, intermetallic compounds, and solutions (both solid solutions and liquid solutions) involving mercury as a major component. Sodium amalgams are often used in reactions as strong reducing agents with better handling properties compared to solid sodium. They are less dangerously reactive toward water and in fact are often used as an aqueous suspension. Sodium amalgam was used as a reagent as early as 1862. A synthesis method was described by J. Alfred Wanklyn in 1866. Structure and compositions No particular formula is assigned to "sodium amalgam". Na5Hg8 and Na3Hg are well defined compounds. In sodium amalgams, the Hg-Hg distances are expanded to around 5 Å vs. about 3 Å for mercury itself. Usually amalgams are classified on the weight percent of sodium. Amalgams with 2% Na are solids at room temperature, whereas some more dilute amalgams remain liquid. Preparation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. A conventional current describes the direction in which positive charges move. Electrons have a negative electrical charge, so the movement of electrons is opposite to that of the conventional current flow. Consequently, the mnemonic ''cathode current departs'' also means that electrons flow ''into'' the device's cathode from the external circuit. For example, the end of a household battery marked with a + (plus) is the cathode. The electrode through which conventional current flows the other way, into the device, is termed an anode. Charge flow Conventional current flows from cathode to anode outside of the cell or device (with electrons moving in the opposite direction), regardless of the cell or device type and operating mode. Cathode polarity with respect to the anode can be positi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |