|
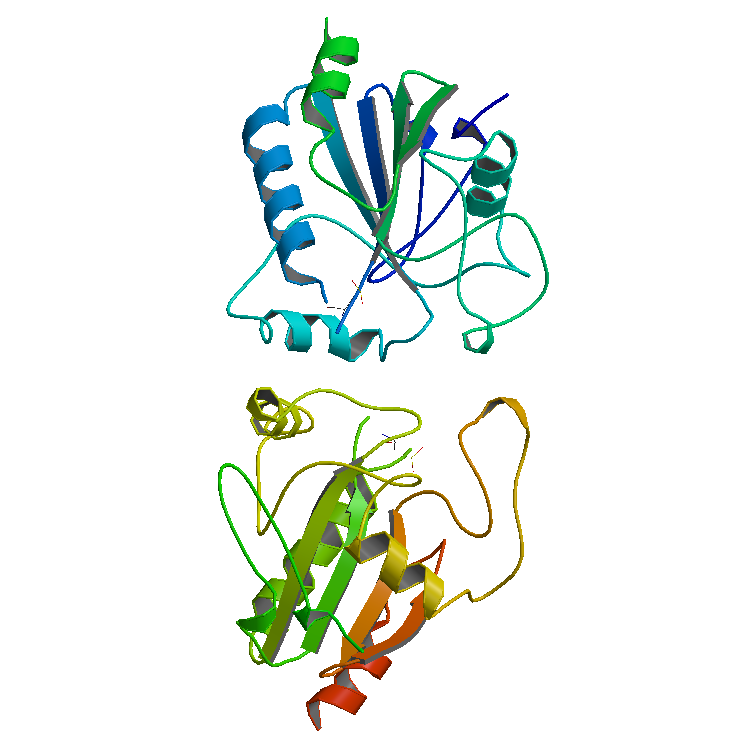
Lactoperoxidase
Lactoperoxidase is a peroxidase enzyme secreted from mammary, salivary and other mucosal glands including the lungs, bronchii and nose that functions as a natural and the first line of defense against bacteria and viruses. Lactoperoxidase is a member of the heme peroxidase family of enzymes. In humans, lactoperoxidase is encoded by the ''LPO'' gene. Lactoperoxidase catalyzes the oxidation of several inorganic and organic substrates by hydrogen peroxide. These substrates include bromide and iodide and therefore lactoperoxidase can be categorised as a haloperoxidase. An other important substrate is thiocyanate. The oxidized products produced through the action of this enzyme have potent and non-specific bactericidal and antiviral activities, including destruction of the influenza virus. Lactoperoxidase together with its inorganic ion substrates, hydrogen peroxide, and oxidized products is known as the lactoperoxidase system. Hence LPO is considered a very important defense again ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver. In biochemical terms, heme is a coordination complex "consisting of an iron ion coordinated to a porphyrin acting as a tetradentate ligand, and to one or two axial ligands." The definition is loose, and many depictions omit the axial ligands. Among the metalloporphyrins deployed by metalloproteins as prosthetic groups, heme is one of the most widely used and defines a family of proteins known as hemoproteins. Hemes are most commonly recognized as components of hemoglobin, the red pigment in blood, but are also found in a number of other biologically important hemoproteins such as myoglobin, cytochromes, catalases, heme peroxidase, and endothelial nitric oxide synthase. The word ''haem'' is derived from Greek ''haima'' meaning "blood". Function Hemoproteins have diverse biological functions incl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypothiocyanite
Hypothiocyanite is the anion SCNsup>− and the conjugate base of hypothiocyanous acid (HOSCN). It is an organic compound part of the thiocyanates as it contains the functional group SCN. It is formed when an oxygen is singly bonded to the thiocyanate group. Hypothiocyanous acid is a fairly weak acid; its acid dissociation constant (p''K''a) is 5.3. Hypothiocyanite is formed by peroxidase catalysis of hydrogen peroxide and thiocyanate: : H2O2 + SCN− → OSCN− + H2O As a bactericide Hypothiocyanite occurs naturally in the antimicrobial immune system of the human respiratory tract in a redox reaction catalyzed by the enzyme lactoperoxidase. It has been researched extensively for its capabilities as an alternative antibiotic as it is harmless to human body cells while being cytotoxic to bacteria. The exact processes for making hypothiocyanite have been patented as such an effective antimicrobial has many commercial applications. Mechanism of action Lactoperoxidase-catalyse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eosinophil Peroxidase
Eosinophil peroxidase is an enzyme found within the eosinophil granulocytes, innate immune cells of humans and mammals. This oxidoreductase protein is encoded by the gene ''EPX'', expressed within these myeloid cells. EPO shares many similarities with its orthologous peroxidases, myeloperoxidase (MPO), lactoperoxidase (LPO), and thyroid peroxidase (TPO). The protein is concentrated in secretory granule (cell biology), granules within eosinophils. Eosinophil peroxidase is a heme peroxidase, its activities including the oxidation of halide ions to bacteriocidal reactive oxygen species, the cationic disruption of bacterial cell walls, and the post-translational modification of protein amino acid residues. The major function of eosinophil peroxidase is to catalysis, catalyze the formation of hypohalous acids from hydrogen peroxide and halide ions in solution. For example: : H2O2 + bromide, Br− → hypobromous acid, HOBr + water, H2O Hypohalous acids formed from halides or pseudohal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haloperoxidase
Haloperoxidases are peroxidases that are able to mediate the oxidation of halides by hydrogen peroxide. Both halides and hydrogen peroxide are widely available in the environment. The Nernst equation shows that hydrogen peroxide can oxidize chloride (E°= 1.36 V), bromide (E°= 1.09 V) and iodide (E°= 0.536 V) from a thermodynamic perspective under natural conditions, i.e., a temperature range of about 0–30 °C and a pH ranging from about 3 (humic soil layer) to about 8 (sea water). Fluoride (E°= 2.87 V) cannot be oxidized by hydrogen peroxide. Classification The table shows the classification of haloperoxidases according to the halides whose oxidation they are able to catalyze. The classification of these enzymes by substrate-usability does not necessarily indicate enzyme substrate ''preference.'' For example, although eosinophil peroxidase is ''able'' to oxidize chloride, it preferentially oxidizes bromide. The mammalian haloperoxidases myeloperoxidase (MPO), lactopero ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myeloperoxidase
Myeloperoxidase (MPO) is a peroxidase enzyme that in humans is encoded by the ''MPO'' gene on chromosome 17. MPO is most abundantly expressed in neutrophil granulocytes (a subtype of white blood cells), and produces hypohalous acids to carry out their antimicrobial activity, including hypochlorous acid, the sodium salt of which is the chemical in bleach. It is a lysosomal protein stored in azurophilic granules of the neutrophil and released into the extracellular space during degranulation. Neutrophil myeloperoxidase has a heme pigment, which causes its green color in secretions rich in neutrophils, such as mucus and sputum. The green color contributed to its outdated name verdoperoxidase. Structure The 150-kDa MPO protein is a cationic heterotetramer consisting of two 15-kDa light chains and two variable-weight glycosylated heavy chains bound to a prosthetic heme group complex with calcium ions, arranged as a homodimer of heterodimers. The light chains are glycosylated a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saliva
Saliva (commonly referred to as spit) is an extracellular fluid produced and secreted by salivary glands in the mouth. In humans, saliva is around 99% water, plus electrolytes, mucus, white blood cells, epithelial cells (from which DNA can be extracted), enzymes (such as lipase and amylase), antimicrobial agents (such as secretory IgA, and lysozymes). The enzymes found in saliva are essential in beginning the process of digestion of dietary starches and fats. These enzymes also play a role in breaking down food particles entrapped within dental crevices, thus protecting teeth from bacterial decay. Saliva also performs a lubricating function, wetting food and permitting the initiation of swallowing, and protecting the oral mucosa from drying out. Various animal species have special uses for saliva that go beyond predigestion. Some swifts use their gummy saliva to build nests. ''Aerodramus'' nests form the basis of bird's nest soup. Cobras, vipers, and certain other membe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiocyanate
Thiocyanate (also known as rhodanide) is the anion . It is the conjugate base of thiocyanic acid. Common derivatives include the colourless salts potassium thiocyanate and sodium thiocyanate. Mercury(II) thiocyanate was formerly used in pyrotechnics. Thiocyanate is analogous to the cyanate ion, , wherein oxygen is replaced by sulfur. is one of the pseudohalides, due to the similarity of its reactions to that of halide ions. Thiocyanate used to be known as rhodanide (from a Greek word for rose) because of the red colour of its complexes with iron. Thiocyanate is produced by the reaction of elemental sulfur or thiosulfate with cyanide: : 8 CN- + S8 -> 8 SCN- : CN- + S2O3^2- -> SCN- + SO3^2- The second reaction is catalyzed by thiosulfate sulfurtransferase, a hepatic mitochondrial enzyme, and by other sulfur transferases, which together are responsible for around 80% of cyanide metabolism in the body. Biological chemistry of thiocyanate in medicine Thiocyanate is known to be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxidase
Peroxidases or peroxide reductases ( EC numberbr>1.11.1.x are a large group of enzymes which play a role in various biological processes. They are named after the fact that they commonly break up peroxides. Functionality Peroxidases typically catalyze a reaction of the form: :ROOR' + \overset + 2H+ -> ce + R'OH Optimal substrates For many of these enzymes the optimal substrate is hydrogen peroxide, but others are more active with organic hydroperoxides such as lipid peroxides. Peroxidases can contain a heme cofactor in their active sites, or alternately redox-active cysteine or selenocysteine residues. The nature of the electron donor is very dependent on the structure of the enzyme. * For example, horseradish peroxidase can use a variety of organic compounds as electron donors and acceptors. Horseradish peroxidase has an accessible active site, and many compounds can reach the site of the reaction. * On the other hand, for an enzyme such as cytochrome c peroxidase, the co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Starch
Starch or amylum is a polymeric carbohydrate consisting of numerous glucose units joined by glycosidic bonds. This polysaccharide is produced by most green plants for energy storage. Worldwide, it is the most common carbohydrate in human diets, and is contained in large amounts in staple foods such as wheat, potatoes, maize (corn), rice, and cassava (manioc). Pure starch is a white, tasteless and odorless powder that is insoluble in cold water or alcohol. It consists of two types of molecules: the linear and helical amylose and the branched amylopectin. Depending on the plant, starch generally contains 20 to 25% amylose and 75 to 80% amylopectin by weight. Glycogen, the energy reserve of animals, is a more highly branched version of amylopectin. In industry, starch is often converted into sugars, for example by malting. These sugars may be fermented to produce ethanol in the manufacture of beer, whisky and biofuel. In addition, sugars produced from processed starch are used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucose Oxidase
The glucose oxidase enzyme (GOx or GOD) also known as notatin (EC number 1.1.3.4) is an oxidoreductase that catalyses the oxidation of glucose to hydrogen peroxide and D-glucono-δ-lactone. This enzyme is produced by certain species of fungi and insects and displays antibacterial activity when oxygen and glucose are present. Glucose oxidase is widely used for the determination of free glucose in body fluids (medical testing), in vegetal raw material, and in the food industry. It also has many applications in biotechnologies, typically enzyme assays for biochemistry including biosensors in nanotechnologies. It was first isolated by Detlev Müller in 1928 from ''Aspergillus niger''. Function Several species of fungi and insects synthesize glucose oxidase, which produces hydrogen peroxide, which kills bacteria. Notatin, extracted from antibacterial cultures of ''Penicillium notatum'', was originally named Penicillin A, but was renamed to avoid confusion with penicillin. Notati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats, as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight, where it is used to make cellulose in cell walls, the most abundant carbohydrate in the world. In energy metabolism, glucose is the most important source of energy in all organisms. Glucose for metabolism is stored as a polymer, in plants mainly as starch and amylopectin, and in animals as glycogen. Glucose circulates in the blood of animals as blood sugar. The naturally occurring form of glucose is -glucose, while -glucose is produced synthetically in comparatively small amounts and is less biologically active. Glucose is a monosaccharide containing six carbon atoms and an aldehyde group, and is therefore an aldohexose. The glucose molecule can exist in an open-chain (acyclic) as well as ring (cyclic) form. Gluco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |