|
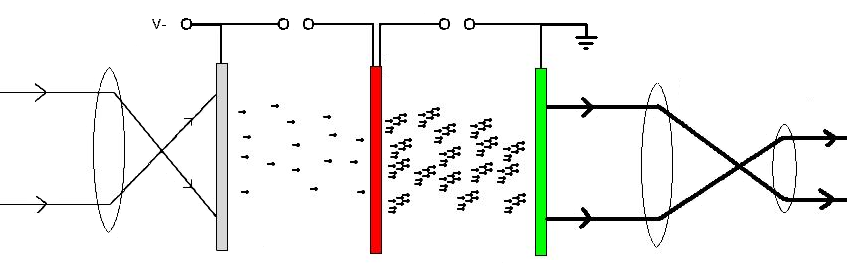
Image Intensification
An image intensifier or image intensifier tube is a vacuum tube device for increasing the intensity of available light in an optical system to allow use under low-light conditions, such as at night, to facilitate visual imaging of low-light processes, such as fluorescence of materials in X-rays or gamma rays (X-ray image intensifier), or for conversion of non-visible light sources, such as near-infrared or short wave infrared to visible. They operate by converting photons of light into electrons, amplifying the electrons (usually with a microchannel plate), and then converting the amplified electrons back into photons for viewing. They are used in devices such as night-vision goggles. Introduction Image intensifier tubes (IITs) are optoelectronic devices that allow many devices, such as night vision devices and medical imaging devices, to function. They convert low levels of light from various wavelengths into visible quantities of light at a single wavelength. Operation Image i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vacuum Tube
A vacuum tube, electron tube, valve (British usage), or tube (North America), is a device that controls electric current flow in a high vacuum between electrodes to which an electric voltage, potential difference has been applied. The type known as a thermionic tube or thermionic valve utilizes thermionic emission of electrons from a hot cathode for fundamental electronic functions such as signal amplifier, amplification and current rectifier, rectification. Non-thermionic types such as a vacuum phototube, however, achieve electron emission through the photoelectric effect, and are used for such purposes as the detection of light intensities. In both types, the electrons are accelerated from the cathode to the anode by the electric field in the tube. The simplest vacuum tube, the diode (i.e. Fleming valve), invented in 1904 by John Ambrose Fleming, contains only a heated electron-emitting cathode and an anode. Electrons can only flow in one direction through the device—fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Avalanche
An electron avalanche is a process in which a number of free electrons in a transmission medium are subjected to strong acceleration by an electric field and subsequently collide with other atoms of the medium, thereby ionizing them (impact ionization). This releases additional electrons which accelerate and collide with further atoms, releasing more electrons—a chain reaction. In a gas, this causes the affected region to become an Electrical resistivity and conductivity, electrically conductive plasma (physics), plasma. The avalanche effect was discovered by John Sealy Townsend in his work between 1897 and 1901, and is also known as the Townsend discharge. Electron avalanches are essential to the dielectric breakdown process within gases. The process can culminate in corona discharges, streamer discharge , streamers, leader (spark), leaders, or in a electric spark, spark or continuous electric arc, arc that completely bridges the gap between the electrical conductors that a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cesium
Caesium (IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that are liquid at or near room temperature. Caesium has physical and chemical properties similar to those of rubidium and potassium. It is pyrophoric and reacts with water even at . It is the least electronegative element, with a value of 0.79 on the Pauling scale. It has only one stable isotope, caesium-133. Caesium is mined mostly from pollucite. The element has 40 known isotopes, making it, along with barium and mercury, one of the elements with the most isotopes. Caesium-137, a fission product, is extracted from waste produced by nuclear reactors. The German chemist Robert Bunsen and physicist Gustav Kirchhoff discovered caesium in 1860 by the newly developed method of flame spectroscopy. The first small-scale applications for caesium were a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cesium Telluride
Caesium (IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that are liquid at or near room temperature. Caesium has physical and chemical properties similar to those of rubidium and potassium. It is pyrophoric and reacts with water even at . It is the least electronegative element, with a value of 0.79 on the Pauling scale. It has only one stable isotope, caesium-133. Caesium is mined mostly from pollucite. The element has 40 known isotopes, making it, along with barium and mercury, one of the elements with the most isotopes. Caesium-137, a fission product, is extracted from waste produced by nuclear reactors. The German chemist Robert Bunsen and physicist Gustav Kirchhoff discovered caesium in 1860 by the newly developed method of flame spectroscopy. The first small-scale applications for caesium were a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nanometer, nm (with a corresponding frequency around 30 Hertz, PHz) to 400 nm (750 Hertz, THz), shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight, and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights. Although long-wavelength ultraviolet is not considered an ionizing radiation because its photons lack the energy to ionization, ionize atoms, it can cause chemical reactions and causes many substances to glow or fluorescence, fluoresce. Consequently, the chemical and biological effects of UV are greater than simple heating effects, and many practical applications of UV radiation derive from its interactions with organic molecules. Short-wave ultraviolet light damages DNA and sterilizes surf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Efficiency
The term quantum efficiency (QE) may apply to incident photon to converted electron (IPCE) ratio of a photosensitive device, or it may refer to the TMR effect of a Magnetic Tunnel Junction. This article deals with the term as a measurement of a device's electrical sensitivity to light. In a charge-coupled device (CCD) or other photodetector, it is the ratio between the number of charge carriers collected at either terminal and the number of photons hitting the device's photoreactive surface. As a ratio, QE is dimensionless, but it is closely related to the responsivity, which is expressed in amps per watt. Since the energy of a photon is inversely proportional to its wavelength, QE is often measured over a range of different wavelengths to characterize a device's efficiency at each photon energy level. For typical semiconductor photodetectors, QE drops to zero for photons whose energy is below the band gap. A photographic film typically has a QE of much less than 10%, while C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caesium
Caesium (IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that are liquid at or near room temperature. Caesium has physical and chemical properties similar to those of rubidium and potassium. It is pyrophoric and reacts with water even at . It is the least electronegative element, with a value of 0.79 on the Pauling scale. It has only one stable isotope, caesium-133. Caesium is mined mostly from pollucite. The element has 40 known isotopes, making it, along with barium and mercury, one of the elements with the most isotopes. Caesium-137, a fission product, is extracted from waste produced by nuclear reactors. The German chemist Robert Bunsen and physicist Gustav Kirchhoff discovered caesium in 1860 by the newly developed method of flame spectroscopy. The first small-scale applications for caesium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats, as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver
Silver is a chemical element with the Symbol (chemistry), symbol Ag (from the Latin ', derived from the Proto-Indo-European wikt:Reconstruction:Proto-Indo-European/h₂erǵ-, ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, white, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. The metal is found in the Earth's crust in the pure, free elemental form ("native silver"), as an alloy with gold and other metals, and in minerals such as argentite and chlorargyrite. Most silver is produced as a byproduct of copper, gold, lead, and zinc Refining (metallurgy), refining. Silver has long been valued as a precious metal. Silver metal is used in many bullion coins, sometimes bimetallism, alongside gold: while it is more abundant than gold, it is much less abundant as a native metal. Its purity is typically measured on a per-mille basis; a 94%-pure alloy is described as "0.940 fine". As one of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
World War II
World War II or the Second World War, often abbreviated as WWII or WW2, was a world war that lasted from 1939 to 1945. It involved the vast majority of the world's countries—including all of the great powers—forming two opposing military alliances: the Allies and the Axis powers. World War II was a total war that directly involved more than 100 million personnel from more than 30 countries. The major participants in the war threw their entire economic, industrial, and scientific capabilities behind the war effort, blurring the distinction between civilian and military resources. Aircraft played a major role in the conflict, enabling the strategic bombing of population centres and deploying the only two nuclear weapons ever used in war. World War II was by far the deadliest conflict in human history; it resulted in 70 to 85 million fatalities, mostly among civilians. Tens of millions died due to genocides (including the Holocaust), starvation, ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Philips
Koninklijke Philips N.V. (), commonly shortened to Philips, is a Dutch multinational conglomerate corporation that was founded in Eindhoven in 1891. Since 1997, it has been mostly headquartered in Amsterdam, though the Benelux headquarters is still in Eindhoven. Philips was formerly one of the largest electronics companies in the world, but is currently focused on the area of health technology, having divested its other divisions. The company was founded in 1891 by Gerard Philips and his father Frederik, with their first products being light bulbs. It currently employs around 80,000 people across 100 countries. The company gained its royal honorary title (hence the ''Koninklijke'') in 1998 and dropped the "Electronics" in its name in 2013, due to its refocusing from consumer electronics to healthcare technology. Philips is organized into three main divisions: Personal Health (formerly Philips Consumer Electronics and Philips Domestic Appliances and Personal Care), Connecte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Netherlands
) , anthem = ( en, "William of Nassau") , image_map = , map_caption = , subdivision_type = Sovereign state , subdivision_name = Kingdom of the Netherlands , established_title = Before independence , established_date = Spanish Netherlands , established_title2 = Act of Abjuration , established_date2 = 26 July 1581 , established_title3 = Peace of Münster , established_date3 = 30 January 1648 , established_title4 = Kingdom established , established_date4 = 16 March 1815 , established_title5 = Liberation Day (Netherlands), Liberation Day , established_date5 = 5 May 1945 , established_title6 = Charter for the Kingdom of the Netherlands, Kingdom Charter , established_date6 = 15 December 1954 , established_title7 = Dissolution of the Netherlands Antilles, Caribbean reorganisation , established_date7 = 10 October 2010 , official_languages = Dutch language, Dutch , languages_type = Regional languages , languages_sub = yes , languages = , languages2_type = Reco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |