|
Isocyanide
An isocyanide (also called isonitrile or carbylamine) is an organic compound with the functional group –. It is the isomer of the related nitrile (–C≡N), hence the prefix is ''isocyano''.IUPAC Goldboo''isocyanides''/ref> The organic fragment is connected to the isocyanide group through the nitrogen atom, not via the carbon. They are used as building blocks for the synthesis of other compounds. Properties Structure and bonding The C-N distance in isocyanides is 115.8 pm in methyl isocyanide. The C-N-C angles are near 180°. Akin to carbon monoxide, isocyanides are described by two Resonance (chemistry), resonance structures, one with a triple bond between the nitrogen and the carbon and one with a double bond between. The π lone pair of the nitrogen stabilizes the structure and is responsible of the linearity of isocyanides, although the reactivity of isocyanides reflects some carbene character, at least in a formal sense. Thus, both resonance structures are useful repres ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Isocyanide
Methyl isocyanide or isocyanomethane is an organic compound and a member of the isocyanide family. This colorless liquid is isomeric and isoelectronic to methyl cyanide (acetonitrile), but its reactivity is very different. In contrast to the faintly sweet, ethereal odor of acetonitrile, the smell of methyl isocyanide, like that of other simple volatile isocyanides, is distinctly penetrating and vile. Methyl isocyanide is mainly used for making 5-membered heterocyclic rings. The C-N distance in methyl isocyanide is very short, 1.158 Å as is characteristic of isocyanides. Preparation and uses Methyl isocyanide was first prepared by A. Gautier by reaction of silver cyanide with methyl iodide. The common method for preparing methyl isocyanides is the dehydration of N-Methylformamide, ''N''-methylformamide. Many metal cyanides react with methylating agents to give complexes of methyl isocyanide. This kind of reactivity has been invoked as being relevant to the origin of life. Methy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
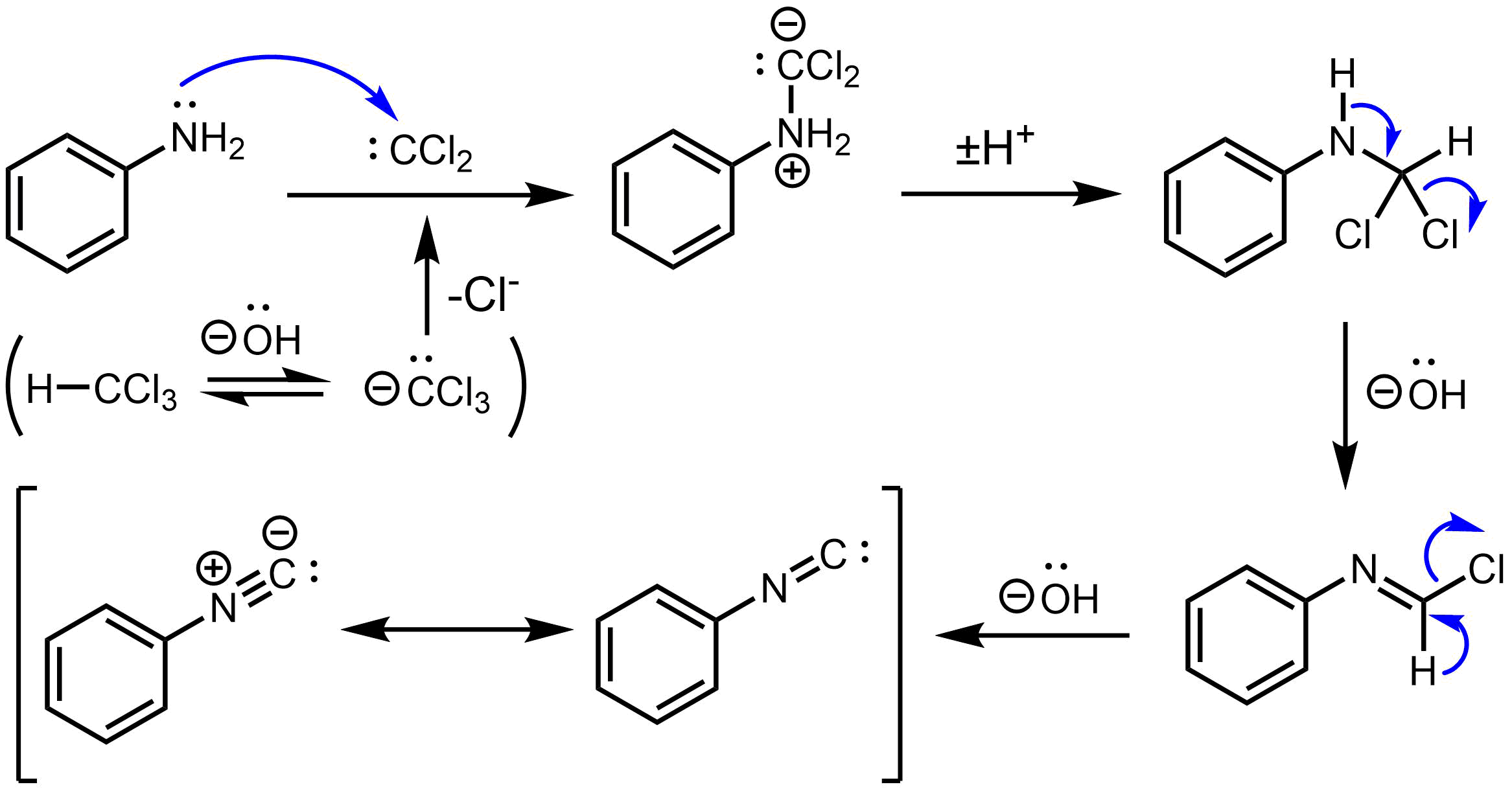
Carbylamine Reaction
The carbylamine reaction (also known as the Hoffmann isocyanide synthesis) is the synthesis of an isocyanide by the reaction of a primary amine, chloroform, and base. The conversion involves the intermediacy of dichlorocarbene. Illustrative is the synthesis of ''tert''-butyl isocyanide from ''tert''-butylamine in the presence of catalytic amount of the phase transfer catalyst benzyltriethylammonium chloride. :Me3CNH2 + CHCl3 + 3 NaOH → Me3CNC + 3 NaCl + 3 H2O Similar reactions have been reported for aniline. It is used to prepare secondary amines. Test for primary amines As it is only effective for primary amines, the carbylamine reaction can be used as a chemical test for their presence. In this context, the reaction is also known as Saytzeff's isocyanide test. In this reaction, the analyte is heated with alcoholic potassium hydroxide and chloroform. If a primary amine is present, the isocyanide An isocyanide (also called isonitrile or carbylamine) is an organic co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tosylmethyl Isocyanide
TosMIC (toluenesulfonylmethyl isocyanide) is an organic compound with the formula CH3C6H4SO2CH2NC. The molecule contains both sulfonyl and isocyanide An isocyanide (also called isonitrile or carbylamine) is an organic compound with the functional group –. It is the isomer of the related nitrile (–C≡N), hence the prefix is ''isocyano''.IUPAC Goldboo''isocyanides''/ref> The organic fragme ... groups. It is a colourless solid that, unlike many isocyanides, is odorless. It is prepared by dehydration of the related formamide derivative. It is used to convert ketones to nitriles ( Van Leusen reaction) and in the preparation of oxazoles. and imidazoles. The versatility of TosMIC in organic synthesis has been documented. It is a fairly strong carbon acid, with an estimated p''K''a of 14 (compared to 29 for methyl tolyl sulfone), the isocyano group acting as an electron acceptor of strength comparable to an ester group. References Further reading * {{cite book , doi=10.1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Cyanide
Hydrogen cyanide (formerly known as prussic acid) is a chemical compound with the chemical formula, formula HCN and structural formula . It is a highly toxic and flammable liquid that boiling, boils slightly above room temperature, at . HCN is produced on an industrial scale and is a highly valued Precursor (chemistry), precursor to many chemical compounds ranging from polymers to pharmaceuticals. Large-scale applications are for the production of potassium cyanide and adiponitrile, used in mining and plastics, respectively. It is more toxic than solid cyanide compounds due to its Volatility (chemistry), volatile nature. A solution of hydrogen cyanide in water (molecule), water, represented as HCN(aqueous, aq), is called ''hydrocyanic acid''. The Salt (chemistry), salts of the cyanide anion are known as cyanides. Whether hydrogen cyanide is an organic compound or not is a topic of debate among chemists, and opinions vary from author to author. Traditionally, it is considered ino ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dichlorocarbene
Dichlorocarbene is the reactive intermediate with chemical formula CCl2. Although this chemical species has not been isolated, it is a common intermediate in organic chemistry, being generated from chloroform. This bent diamagnetic molecule rapidly inserts into other bonds. Preparation Dichlorocarbene is most commonly generated by reaction of chloroform and a base such as potassium tert-butoxide, potassium ''tert''-butoxide or aqueous sodium hydroxide. A phase transfer catalyst, for instance quaternary ammonium cation, benzyltriethylammonium bromide, facilitates the migration of the hydroxide in the organic phase. :HCCl3 + NaOH → CCl2 + NaCl + H2O Other reagents and routes Another precursor to dichlorocarbene is ethyl trichloroacetate. Upon treatment with sodium methoxide it releases CCl2. Phenyl(trichloromethyl)mercury decomposes thermally to release CCl2. :PhHgCCl3 → CCl2 + PhHgCl Dichlorodiazirine, which is stable in the dark, chemical decomposition, decomposes into dic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The Reactivity (chemistry), reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive Chemical property, chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their Chemical polarity, nonp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diphosgene
Diphosgene is an organic chemical compound with the formula ClCO2CCl3. This colorless liquid is a valuable reagent in the synthesis of organic compounds. Diphosgene is related to phosgene and has comparable toxicity, but is more conveniently handled because it is a liquid, whereas phosgene is a gas. Production and uses Diphosgene is prepared by radical chlorination of methyl chloroformate under UV light: :Cl-CO-OCH3 + 3 Cl2 —(hv)→ Cl-CO-OCCl3 + 3 HCl Another method is the radical chlorination of methyl formate: :H-CO-OCH3 + 4 Cl2 —(hv)→ Cl-CO-OCCl3 + 4 HCl Diphosgene converts to phosgene upon heating or upon catalysis with charcoal. It is thus useful for reactions traditionally relying on phosgene. For example, it convert amines into isocyanates, secondary amines into carbamoyl chlorides, carboxylic acids into acid chlorides, and formamides into isocyanides. Diphosgene serves as a source of two equivalents of phosgene: :2 RNH2 + ClCO2CCl3 → 2 RNCO + 4 HC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ivar Karl Ugi
Ivar Karl Ugi (9 September 1930 in Saaremaa, Estonia – 29 September 2005 in Munich) was an Estonian-born German chemist who made major contributions to organic chemistry. He is known for the research on multicomponent reactions, yielding the Ugi reaction. Biography After he went to Germany from Estonia in 1941 he began his studies of chemistry in 1949 at the University of Tübingen until 1951. He became Dr. rer. nat. in 1954 at the Ludwig Maximilian University of Munich. He did his habilitation 1960 at the same university. After a short but very successful career in industry at Bayer from 1962 until 1968 when he joined the University of Southern California at Los Angeles. From 1971 he worked at the Technical University of Munich, and was an emeritus from 1999 until his death in 2005. Research and development The one pot reaction of a ketone or aldehyde, an amine, an isocyanide and a carboxylic acid to form a bis-amide In organic chemistry, an amide, also known as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ritter Reaction
The Ritter reaction (sometimes called the Ritter amidation) is a chemical reaction that transforms a nitrile into an ''N''-alkyl amide using various electrophilic alkylating reagents. The original reaction formed the alkylation, alkylating agent using an alkene in the presence of a strong acid. Mechanism and scope The Ritter reaction proceeds by the electrophilic addition of either a carbenium ion or covalent species to the nitrile. The resulting nitrilium ion is hydrolysis, hydrolyzed to the desired amide. Primary, secondary, tertiary, and benzylic alcohol (chemistry), alcohols, as well as ''tert''-butyl acetate, also successfully react with nitriles in the presence of strong acids to form amides via the Ritter reaction. A wide range of nitriles can be used. In particular, cyanide can be used to prepare formamides, which are useful precursors to isocyanides, or may also be hydrolysed to give amines. Applications A large scale application of the Ritter reaction is in the sy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triple Bond
A triple bond in chemistry is a chemical bond between two atoms involving six Electron pair bond, bonding electrons instead of the usual two in a covalent bond, covalent single bond. Triple bonds are stronger than the equivalent covalent bond, single bonds or double bond, double bonds, with a bond order of three. The most common triple bond is in a nitrogen N2 molecule; the second most common is that between two carbon atoms, which can be found in alkynes. Other functional groups containing a triple bond are cyanides and isocyanides. Some diatomic molecules, such as diphosphorus and carbon monoxide, are also triple bonded. In skeletal formula, skeletal formulae the triple bond is drawn as three parallel lines (≡) between the two connected atoms. Bonding Triple bonding can be explained in terms of orbital hybridization. In the case of acetylene, each carbon atom has two sp orbital, sp-orbitals and two p-orbitals. The two sp-orbitals are linear, with 180° bond angles, and occupy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toluenesulfonyl Chloride
4-Toluenesulfonyl chloride (''p''-toluenesulfonyl chloride, toluene-''p''-sulfonyl chloride) is an organic compound with the formula CH3C6H4SO2Cl. This white, malodorous solid is a reagent widely used in organic synthesis. Abbreviated TsCl or TosCl, it is a derivative of toluene and contains a sulfonyl chloride (−SO2Cl) functional group. Uses As typical for Sulfonyl halides, TsCl converts alcohols (abbreviated ROH) into the corresponding toluenesulfonate esters, or tosyl derivatives ("tosylates"): : CH3C6H4SO2Cl + ROH → CH3C6H4SO2OR + HCl Tosylates can be cleaved with lithium aluminium hydride: : 4 CH3C6H4SO2OR + LiAlH4 → LiAl(O3SC6H4CH3)4 + 4 RH Thus, tosylation followed by reduction allows for removal of a hydroxyl group. Likewise, TsCl is used to prepare sulfonamides from amines: :CH3C6H4SO2Cl + R2NH → CH3C6H4SO2NR2 + HCl The resulting sulfonamides are non-basic and, when derived from primary amines, are even acidic. TsCl reacts with hydrazine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Oxychloride
Phosphoryl chloride (commonly called phosphorus oxychloride) is a colourless liquid with the formula . It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride. It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide. It is mainly used to make phosphate esters. Structure Like phosphate, is tetrahedral in shape. It features three P−Cl bonds and one strong P–O bond, with an estimated bond dissociation energy of 533.5 kJ/mol. Unlike in the case of , the Schomaker-Stevenson rule predicts appropriate bond length for the P–O bond only if the P–O bond is treated as a double bond, P=O. More modern treatments explain the tight P–O bond as a combination of lone pair transfer from the phosphorus to the oxygen atom and a dative ''π'' back-bond that produces an effective + −configuration. Phosphoryl chloride exists as neutral molecules in the solid, liquid and gas states. This is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |