|
Intimate Ion Pair
In chemistry, the intimate ion pair concept, introduced by Saul Winstein, describes the interactions between a cation, anion and surrounding solvent molecules. In ordinary aqueous solutions of inorganic salts, an ion is completely solvated and shielded from the counterion. In less polar solvents, two ions can still be connected to some extent. In a ''tight'', ''intimate'', or ''contact'' ion pair, there are no solvent molecules between the two ions. When solvation increases, ionic bonding decreases and a ''loose'' or ''solvent-shared'' ion pair results. The ion pair concept explains stereochemistry in solvolysis. The concept of intimate ion pairs is used to explain the slight tendency for inversion of stereochemistry during an S1 reaction. It is proposed that solvent or other ions in solution may assist in the removal of a leaving group to form a carbocation which reacts in an S1 fashion; similarly, the leaving group may associate loosely with the cationic intermediate. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during chemical reaction, reactions with other chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
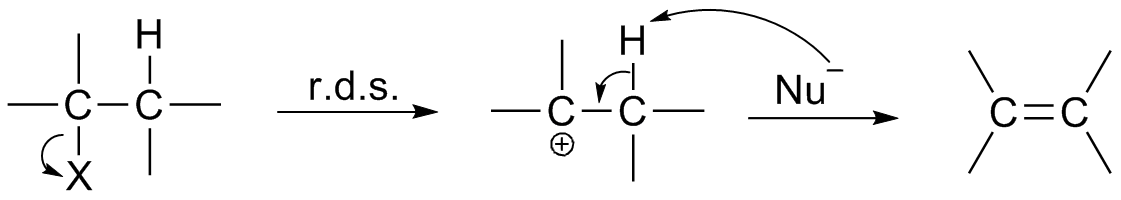
Solvolysis
In chemistry, solvolysis is a type of nucleophilic substitution (S1/S2) or elimination reaction, elimination where the nucleophile is a solvent molecule. Characteristic of S1 reactions, solvolysis of a chirality (chemistry), chiral reactant affords the racemate. Sometimes however, the stereochemical course is complicated by intimate ion pairs, whereby the leaving anion remains close to the carbocation, effectively shielding it from an attack by the nucleophile. Particularly fast reactions can occur by neighbour group participation, with nonclassical ions as intermediates or transition states. Examples For certain nucleophiles, solvolysis reactions are classified. Solvolysis involving water (molecule), water is called hydrolysis. Related terms are alcoholysis (Alcohol (chemistry), alcohols) and specifically methanolysis (methanol), acetolysis, ammonolysis (ammonia), and aminolysis (alkyl amines). Glycolysis is however an older term for the multistep conversion of glucose to pyruvat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Asymmetric Ion-pairing Catalysis
Asymmetric ion-pairing catalysis in chemistry is a type of asymmetric catalysis taking place specifically with charged intermediates or charged reagents. In one type of catalysis ion-pairing exists with a charged and chiral catalyst. The charged catalyst can be cationic or anionic. Catalysis by anionic catalysts is also called asymmetric counteranion-directed catalysis. In the other variation of asymmetric ion-pairing catalysis called anion or cation binding, the chiral catalyst is neutral but binds in a noncovalent way to an intermediate ion pair. Asymmetric ion-pairing catalysis is distinct from other covalent types of catalysis such as Lewis acid catalysis and Bronsted acid catalysis. It is one of several strategies in enantioselective synthesis and of some relevance to academic research. The first reported example of this type of catalysis is attributed to Dolling, Davis & Grabowski who in 1984 used a chiral cinchonidine based phase transfer catalyst in the synthesis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion Association
In chemistry, ion association is a chemical reaction whereby ions of opposite electric charge come together in solution to form a distinct chemical entity. Ion associates are classified, according to the number of ions that associate with each other, as ''ion pairs'', ''ion triplets'', etc. ''Intimate ion pairs'' are also classified according to the nature of the interaction as contact, solvent-shared or solvent-separated. The most important factor to determine the extent of ion association is the dielectric constant of the solvent. Ion associates have been characterized by means of vibrational spectroscopy, as introduced by Niels Bjerrum, and dielectric-loss spectroscopy. Classification of ion pairs ''Ion pairs'' are formed when a cation and anion, which are present in a solution of an ionizable substance, come together to form a discrete chemical species. There are three distinct types of ''ion pairs'', depending on the extent of solvation of the two ions. For example, ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Racemic
In chemistry, a racemic mixture or racemate () is a mixture that has equal amounts (50:50) of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. History The first known racemic mixture was racemic acid, which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid. He manually separated the crystals of a mixture, starting from an aqueous solution of the sodium ammonium salt of racemate tartaric acid. Pasteur benefited from the fact that ammonium tartrate salt gives enantiomeric crystals with distinct crystal forms (at 77 °F). Reasoning from the macroscopic scale down to the molecular, he reckoned that the molecules had to have non-superimposable mirror images. A sample with only a single enantiomer is an ''enantiomerically pure'' or ''enantiopure'' compound. Etymology The word ''racemic'' derives from Latin , meaning pertaining to a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are Lewis bases. ''Nucleophilic'' describes the affinity of a nucleophile to bond with positively charged Atomic nucleus, atomic nuclei. Nucleophilicity, sometimes referred to as nucleophile strength, refers to a substance's nucleophilic character and is often used to compare the affinity of atoms. Neutral nucleophilic reactions with solvents such as Alcohol (chemistry), alcohols and water are named solvolysis. Nucleophiles may take part in nucleophilic substitution, whereby a nucleophile becomes attracted to a full or partial positive charge, and nucleophilic addition. Nucleophilicity is closely related to basicity. The difference between the two is, that basicity is a thermodynamic property (i.e. relates to an equilibrium state), but nucleop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Intermediate
In chemistry, a reaction intermediate, or intermediate, is a molecular entity arising within the sequence of a stepwise chemical reaction. It is formed as the reaction product of an elementary step, from the reactants and/or preceding intermediates, but is consumed in a later step. It does not appear in the chemical equation for the overall reaction. For example, consider this hypothetical reaction: :A + B → C + D If this overall reaction comprises two elementary steps thus: :A + B → X :X → C + D then X is a reaction intermediate. The phrase ''reaction intermediate'' is often abbreviated to the single word ''intermediate'', and this is IUPAC's preferred form of the term. But this shorter form has other uses. It often refers to reactive intermediates. It is also used more widely for chemicals such as cumene which are traded within the chemical industry but are not generally of value outside it. IUPAC definition The IUPAC Gold Book defines an ''intermediate'' as a co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further classified in two main categories according to the coordination number of the charged carbon: three in the carbenium ions and five in the carbonium ions. Among the simplest carbocations are the methenium (a carbenium ion), methanium (a carbonium ion), acylium ions , and Vinyl cation, vinyl cations. Until the early 1970s, carbocations were called ''carbonium ions''. This nomenclature was proposed by George Andrew Olah, G. A. Olah. Carbonium ions, as originally defined by Olah, are characterized by a Three-center two-electron bond, three-center two-electron delocalized bonding scheme and are essentially synonymous with so-called 'non-classical carbocations', which are carbocations that contain bridging C–C or C–H σ-bonds. However ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SN1 Reaction
The unimolecular nucleophilic substitution (SN1) reaction is a substitution reaction in organic chemistry. The Hughes-Ingold symbol of the mechanism expresses two properties—"SN" stands for "nucleophilic substitution", and the "1" says that the rate-determining step is molecularity, unimolecular. Thus, the rate equation is often shown as having first-order dependence on the substrate and zero-order dependence on the nucleophile. This relationship holds for situations where the amount of nucleophile is much greater than that of the intermediate. Instead, the rate equation may be more accurately described using Steady state (chemistry), steady-state kinetics. The reaction involves a carbocation intermediate and is commonly seen in reactions of secondary or tertiary alkyl halides under strongly basic conditions or, under strongly acidic conditions, with Alcohol (chemistry), secondary or tertiary alcohols. With primary and secondary alkyl halides, the alternative SN2 reaction, SN2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, studies the spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which are defined as having the same molecular formula and sequence of bonded atoms (constitution) but differing in the geometric positioning of the atoms in space. For this reason, it is also known as Three-dimensional space, 3D chemistry—the prefix "stereo-" means "three-dimensionality". Stereochemistry applies to all kinds of compounds and ions, Organic chemistry, organic and Inorganic chemistry, inorganic species alike. Stereochemistry affects Biochemistry, biological, Physical chemistry, physical, and supramolecular chemistry. Stereochemistry reactivity (chemistry), reactivity of the molecules in question (dynamic stereochemistry). History In 1815, Jean-Baptiste Biot's observation of optical activity marked the beginning of organic stereochemistr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saul Winstein
Saul Winstein (October 8, 1912 – November 23, 1969) was a Jewish Canadian chemist who discovered the ''Winstein reaction.'' He argued a non-classical cation was needed to explain the stability of the norbornyl cation. This fueled a debate with Herbert C. Brown over the existence of σ-delocalized carbocations. Winstein also first proposed the concept of an intimate ion pair. He was co-author of the Grunwald–Winstein equation, concerning solvolysis rates. Richard F. Heck, who earlier in his career had undertaken postgraduate studies with Winstein, won the 2010 Nobel Prize in Chemistry. References External links UCLA Biography 1912 births 1969 deaths Jewish Canadian scientists Jewish chemists Canadian chemists National Medal of Science laureates Canadian expatriates in the United States {{Chemist-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |