|
Hydroxymethylfurfural
Hydroxymethylfurfural (HMF), also 5-(hydroxymethyl)furfural, is an organic compound formed by the dehydration of reducing sugars. It is a white low-melting solid (although commercial samples are often yellow) which is highly soluble in both water and organic solvents. The molecule consists of a furan ring, containing both aldehyde and alcohol functional groups. HMF can form in sugar-containing food, particularly as a result of heating or cooking. Its formation has been the topic of significant study as HMF was regarded as being potentially carcinogenic to humans. However, so far in vivo genotoxicity was negative. No relevance for humans concerning carcinogenic and genotoxic effects can be derived. HMF is classified as a food improvement agent and is primarily being used in the food industry in form of a food additive as a biomarker as well as a flavoring agent for food products. It is also produced industrially on a modest scale as a carbon-neutral feedstock for the production o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,5-Furandicarboxylic Acid
2,5-Furandicarboxylic acid (FDCA) is an organic chemical compound consisting of two carboxylic acid groups attached to a central furan ring. It was first reported as ''dehydromucic acid'' by Rudolph Fittig and Heinzelmann in 1876, who produced it via the action of concentrated hydrobromic acid upon mucic acid. It can be produced from certain carbohydrates and as such is a renewable resource, it was identified by the US Department of Energy as one of 12 priority chemicals for establishing the “green” chemistry industry of the future. Furan-2,5-dicarboxylic acid (FDCA) has been suggested as an important renewable building block because it can substitute for terephthalic acid (PTA) in the production of polyesters and other current polymers containing an aromatic moiety.T. Werpy, G. Petersen: ''Top Value Added Chemicals from Biomass. Volume I – Results of Screening for Potential Candidates from Sugars and Synthesis Gas.'' Produced by the Staff at Pacific Northwest National Labora ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methoxymethylfurfural
Methoxymethylfurfural (MMF or 5-methoxymethylfuran-2-carbaldehyde) is an organic compound derived from dehydration of sugars and subsequent etherification with methanol.van Putten, R-J., van der Waal J.C. de Jong, E., Rasrendra C.B., Heeres, E.J. and de Vries HG. (2011) Furan-based platform chemicals of the future. Dehydration of hexoses as biosustainable product route. Chemical Reviews submitted. This colorless liquid is soluble in a wide range of solvents including lower alcohols. The molecule is a derivative of furan, containing both aldehyde and ether (methoxymethyl) functional groups. MMF has been detected in the leaves and roots of Chilean ''Jaborosa magellanica'' (Solanaceae). It has a typical odor suggestive of maraschino cherries.Hind, J.D. and Crayton, F.H. (1963) Tobacco flavorants. US 3,095,882 MMF can be made from a wide range of carbohydrate containing feedstocks including sugar, starch and cellulose using a chemical catalytic process and is a potential "carbon-neutr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fructose
Fructose, or fruit sugar, is a Ketose, ketonic monosaccharide, simple sugar found in many plants, where it is often bonded to glucose to form the disaccharide sucrose. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed by the gut directly into the blood of the portal vein during digestion. The liver then converts both fructose and galactose into glucose, so that dissolved glucose, known as blood sugar, is the only monosaccharide present in circulating blood. Fructose was discovered by French chemist Augustin-Pierre Dubrunfaut in 1847. The name "fructose" was coined in 1857 by the English chemist William Allen Miller. Pure, dry fructose is a sweet, white, odorless, crystalline solid, and is the most water-soluble of all the sugars. Fructose is found in honey, tree and vine fruits, flowers, Berry, berries, and most List of root vegetables, root vegetables. Commercially, fructose is derived from sugar cane, sugar beets, and maize. Hi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,5-dimethylfuran
2,5-Dimethylfuran is a heterocyclic compound with the formula (CH3)2C4H2O. Although often abbreviated DMF, it should not be confused with dimethylformamide. A derivative of furan, this simple compound is a potential biofuel, being derivable from cellulose. Production Fructose can be converted into 2,5-dimethylfuran in a catalytic biomass-to-liquid process. The conversion of fructose to DMF proceeds via hydroxymethylfurfural. Fructose is obtainable from glucose, a building block in cellulose. Potential as a biofuel DMF has a number of attractions as a biofuel. It has an energy density 40% greater than that of ethanol, making it comparable to gasoline (petrol). It is also chemically stable and, being insoluble in water, does not absorb moisture from the atmosphere. Evaporating dimethylfuran during the production process also requires around one third less energy than the evaporation of ethanol, although it has a boiling point some 14 °C higher, at 92 °C, compared to 78& ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-Chloromethylfurfural
5-Chloromethylfurfural is an organic compound with the formula C4H2O(CH2Cl)CHO. It consists of a furan substituted at the 2- and 5-positions with formyl (CHO) and chloromethyl (CH2Cl) groups. CMF, as it is called, is obtained by dehydration of fructose and other cellulose derivatives using hydrochloric acid. It is a colourless liquid.I. J. Rinkes "5-Methylfurfural" Org. Synth. 1934, volume 14, 62. It can be reduced to give 5-methylfurfural, and can be hydrolyzed to give hydroxymethylfurfural Hydroxymethylfurfural (HMF), also 5-(hydroxymethyl)furfural, is an organic compound formed by the dehydration of reducing sugars. It is a white low-melting solid (although commercial samples are often yellow) which is highly soluble in both water .... References {{DEFAULTSORT:Chloromethylfurfural, 5- Organochlorides Furfurals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Furfural
Furfural is an organic compound with the formula C4H3OCHO. It is a colorless liquid, although commercial samples are often brown. It has an aldehyde group attached to the 2-position of furan. It is a product of the dehydration of sugars, as occurs in a variety of agricultural byproducts, including corncobs, oat, wheat bran, and sawdust. The name ''furfural'' comes from the Latin word , meaning bran, referring to its usual source. Furfural is only derived from lignocellulosic biomass, i.e., its origin is non-food or non-coal/oil based. Aside from ethanol, acetic acid, and sugar, it is one of the oldest renewable chemicals. It is also found in many processed foods and beverages. History Furfural was first isolated in 1821 (published in 1832) by the German chemist Johann Wolfgang Döbereiner, who produced a small sample as a byproduct of formic acid synthesis. In 1840, the Scottish chemist John Stenhouse found that the same chemical could be produced by distilling a wide variety of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
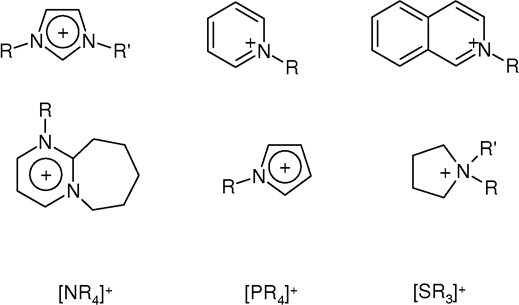
Ionic Liquids
An ionic liquid (IL) is a salt in the liquid state. In some contexts, the term has been restricted to salts whose melting point is below a specific temperature, such as . While ordinary liquids such as water and gasoline are predominantly made of electrically neutral molecules, ionic liquids are largely made of ions. These substances are variously called liquid electrolytes, ionic melts, ionic fluids, fused salts, liquid salts, or ionic glasses. Ionic liquids have many potential applications. They are powerful solvents and can be used as electrolytes. Salts that are liquid at near-ambient temperature are important for electric battery applications, and have been considered as sealants due to their very low vapor pressure. Any salt that melts without decomposing or vaporizing usually yields an ionic liquid. Sodium chloride (NaCl), for example, melts at into a liquid that consists largely of sodium cations () and chloride anions (). Conversely, when an ionic liquid is cooled, it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society (professional association) in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 34,000 in the UK and a further 8,000 abroad. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactive Distillation
Reactive may refer to: *Generally, capable of having a reaction (other) *An adjective abbreviation denoting a bowling ball coverstock made of reactive resin *Reactivity (chemistry) *Reactive mind *Reactive programming See also *Reactance (other) Reactance may refer to: * Electrical reactance In electrical circuits, reactance is the opposition presented to alternating current by inductance or capacitance. Greater reactance gives smaller current for the same applied voltage. Reactance is ... * Reactivity (other) {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid Catalysis
In acid catalysis and base catalysis, a chemical reaction is catalyzed by an acid or a base. By Brønsted–Lowry acid–base theory, the acid is the proton (hydrogen ion, H+) donor and the base is the proton acceptor. Typical reactions catalyzed by proton transfer are esterifications and aldol reactions. In these reactions, the conjugate acid of the carbonyl group is a better electrophile than the neutral carbonyl group itself. Depending on the chemical species that act as the acid or base, catalytic mechanisms can be classified as either specific catalysis and general catalysis. Many enzymes operate by general catalysis. Applications and examples Brønsted acids Acid catalysis is mainly used for organic chemical reactions. Many acids can function as sources for the protons. Acid used for acid catalysis include hydrofluoric acid (in the alkylation process), phosphoric acid, toluenesulfonic acid, polystyrene sulfonate, heteropoly acids, zeolites. Strong acids catalyze the hydr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |