|
Hexamethylenediamine
Hexamethylenediamine is the organic compound with the formula H2N(CH2)6NH2. The molecule is a diamine, consisting of a hexamethylene hydrocarbon chain terminated with amine functional groups. The colorless solid (yellowish for some commercial samples) has a strong amine odor. About 1 billion kilograms are produced annually.Robert A. Smiley "Hexamethylenediamine" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. Synthesis Hexamethylenediamine was first reported by Theodor Curtius. It is produced by the hydrogenation of adiponitrile: :NC(CH2)4CN + 4 H2 → H2N(CH2)6NH2 The hydrogenation is conducted on molten adiponitrile diluted with ammonia, typical catalysts being based on cobalt and iron. The yield is good, but commercially significant side products are generated by virtue of reactivity of partially hydrogenated intermediates. These other products include 1,2-diaminocyclohexane, hexamethyleneimine, and the triamine bis(hexamethylenetriam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nylon 66
Nylon 66 (loosely written nylon 6-6, nylon 6/6, nylon 6,6, or nylon 6:6) is a type of polyamide or nylon. It, and nylon 6, are the two most common for textile and plastic industries. Nylon 66 is made of two monomers each containing 6 carbon atoms, hexamethylenediamine and adipic acid, which give nylon 66 its name. Aside from its superior physical characteristics, nylon 66 is attractive because its precursors are inexpensive. Synthesis and manufacturing Hexamethylenediamine (top) and adipic acid (bottom), monomers used for polycondensation of Nylon 66. Nylon 66 is synthesized by polycondensation of hexamethylenediamine and adipic acid. Equivalent amounts of hexamethylenediamine and adipic acid are combined in water. In the original implementation, the resulting ammonium/ carboxylate salt was isolated and then heated either in batches or continuously to induce polycondensation.n(HOOC - (CH2)4 - COOH) + n(H2N - (CH2)6 - NH2) -> OC - (CH2)4 - CO - NH - (CH2)6 - NH - n + (2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamine
A diamine is an amine with exactly two amino groups. Diamines are used as monomers to prepare polyamides, polyimides, and polyureas. The term ''diamine'' refers mostly to primary diamines, as those are the most reactive. In terms of quantities produced, 1,6-diaminohexane (a precursor to Nylon 6-6) is most important, followed by ethylenediamine. Vicinal diamines (1,2-diamines) are a structural motif in many biological compounds and are used as ligands in coordination chemistry. Aliphatic diamines Linear * 1 carbon: methylenediamine (diaminomethane) of theoretical interest only * 2 carbons: ethylenediamine (1,2-diaminoethane). Related derivatives include the N-alkylated compounds, 1,1-dimethylethylenediamine, 1,2-dimethylethylenediamine, ethambutol, tetrakis(dimethylamino)ethylene, TMEDA. File:Ethylene_diamine.png, Ethylenediamine * 3 carbons: 1,3-diaminopropane (propane-1,3-diamine) * 4 carbons: putrescine (butane-1,4-diamine) * 5 carbons: cadaverine (pentane-1,5-diam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentylamine
1-Aminopentane is an organic compound with the formula CH3(CH2)4NH2. It is used as a solvent, as a raw material in the manufacture of a variety of other compounds, including dyes, emulsifiers, and pharmaceutical products, and as a flavoring agent.Karsten Eller, Erhard Henkes, Roland Rossbacher, Hartmut Höke, "Amines, Aliphatic" Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. Pentylamine exhibits reactions typical of other simple alkyl amines, i.e. protonation, alkylation, acylation, condensation with carbonyls. Like other simple aliphatic In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane ... amines, pentylamine is a weak base: the pKa of H3(CH2)4NH3sup>+ is 10.21. See also * 3-Aminopentane References {{DEFAULTSORT:Aminopentane, 1- Alkylamines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-Methylpentamethylenediamine
2-Methylpentamethylenediamine is an organic compound part of the amine family with the formula H2NCH2CH2CH2CH(CH3)CCH2NCH2. A colorless liquid, this diamine is obtained by the hydrogenation of 2-methylglutaronitrile. Uses 2-Methylpentamethylenediamine serves as a curing agent. It gives good adhesion for metals and resistance against corrosion and other chemicals. It provides toughness, low blush, uniform finish, high gloss, and improves UV stability. It reduces gel time and is compatible with epoxy resins. It is suitable for marine, industrial, and decorative coatings. 2-Methylpentamethylenediamine can also be used as a chain extender for polyurethane applications. Its derivatives like aspartic esters, secondary amines, aldimines and ketoimines serve as curatives in systems of polyurea. In polyamides, 2-Methylpentamethylenediamine acts as a crystallinity disruptor. This makes polymers amorphous in structure and slows down crystallization. It lowers melting point, improves ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
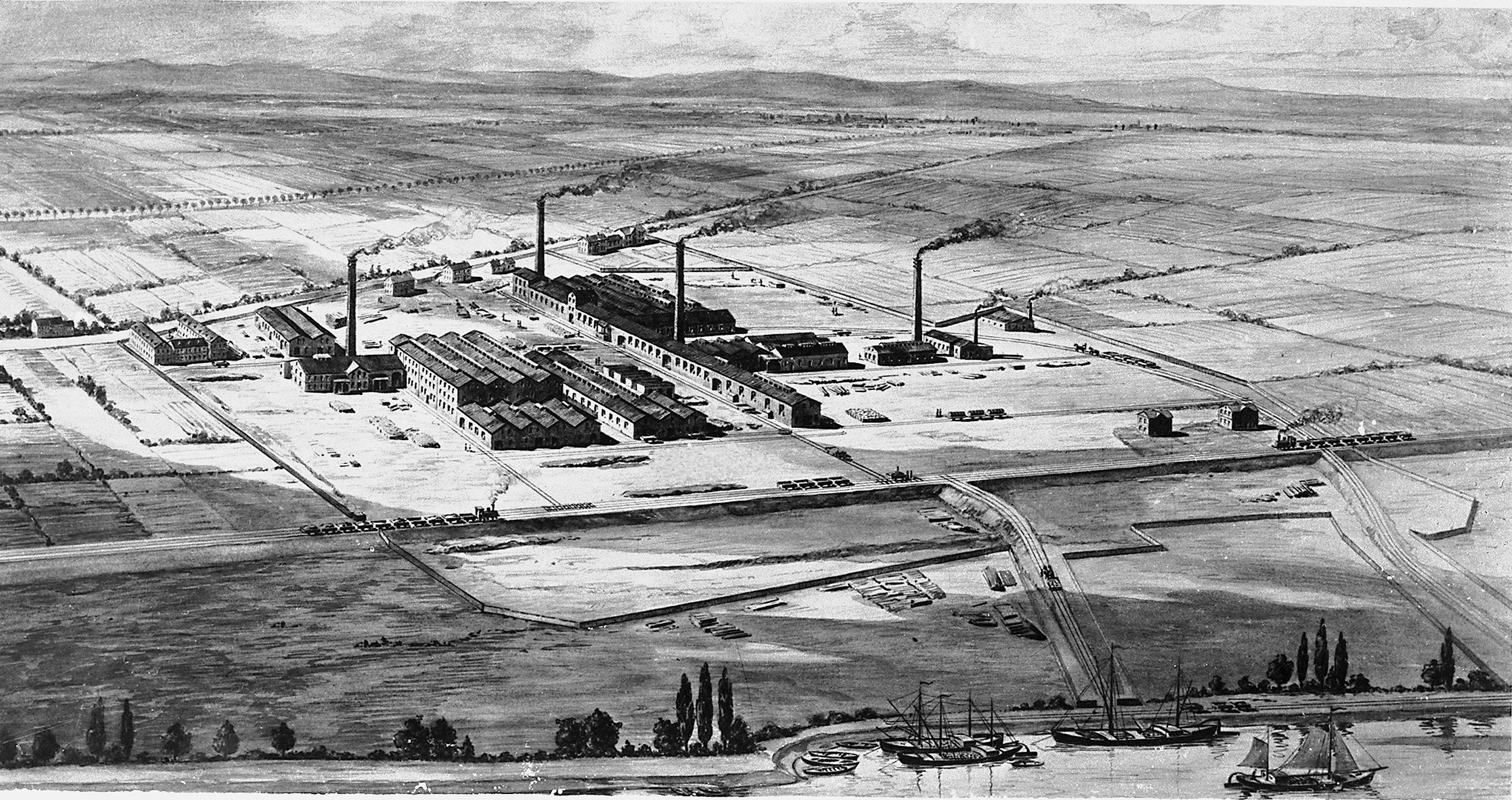
Billingham
Billingham is a town and civil parish in the Borough of Stockton-on-Tees, County Durham, England. The town is on the north side of the River Tees and is governed by Stockton-on-Tees Borough Council. The settlement had previously formed its own borough but was overshadowed by its neighbour. The town had a population of 35,165 at the 2011 Census. The town was founded circa. 650 by a group of Angles known as Billa's people,This is Billingham which is where the name Billingham is thought to have originated. In modern history, the , and in particular the company [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Seal Sands
Seal Sands () is a 294.37 hectare biological Site of Special Scientific Interest in County Durham, England, notified in 1966. Situated in the mouth of the River Tees next to Greatham Creek and Seaton-on-Tees Channel, the site is accessible from the A178 road running between Seaton Carew and Port Clarence. SSSIs are designated by Natural England, formerly English Nature, which uses the 1974–1996 county system. This means there is no grouping of SSSIs by Stockton-on-Tees unitary authority, or County Durham which is the relevant ceremonial county. As such Seal Sands is one of 18 SSSIs in the Cleveland area of search. An area of reclaimed land is given over to chemical industries. Chemical industries site On land recovered from the sea, an area of Seal Sands is used as an industrial park for the chemical industry. Members of the Northeast of England Process Industry Cluster (NEPIC) using the site include: Ineos, Fine Organics, Central Area Transmission System ( BP Gr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BASF
BASF SE () is a German multinational chemical company and the largest chemical producer in the world. Its headquarters is located in Ludwigshafen, Germany. The BASF Group comprises subsidiaries and joint ventures in more than 80 countries and operates six integrated production sites and 390 other production sites in Europe, Asia, Australia, the Americas and Africa. BASF has customers in over 190 countries and supplies products to a wide variety of industries. Despite its size and global presence, BASF has received relatively little public attention since it abandoned the manufacture and sale of BASF-branded consumer electronics products in the 1990s. At the end of 2019, the company employed 117,628 people, with over 54,000 in Germany. , BASF posted sales of €59.3 billion and income from operations before special items of about €4.5 billion. Between 1990 and 2005, the company invested €5.6 billion in Asia, specifically in sites near Nanjing and Shanghai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epoxy Resin
Epoxy is the family of basic components or Curing (chemistry), cured end products of epoxy resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide functional group is also collectively called ''epoxy''. The IUPAC name for an epoxide group is an oxirane. Epoxy resins may be reacted (cross-linked) either with themselves through catalytic homopolymerisation, or with a wide range of co-reactants including polyfunctional amines, acids (and acid anhydrides), phenols, alcohols and thiols (usually called mercaptans). These co-reactants are often referred to as hardeners or curatives, and the cross-linking reaction is commonly referred to as curing. Reaction of polyepoxides with themselves or with polyfunctional hardeners forms a thermosetting polymer, often with favorable mechanical properties and high thermal and chemical resistance. Epoxy has a wide range of applications, including metal coatings, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cross-link
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural polymers (such as proteins). In polymer chemistry "cross-linking" usually refers to the use of cross-links to promote a change in the polymers' physical properties. When "crosslinking" is used in the biological field, it refers to the use of a probe to link proteins together to check for protein–protein interactions, as well as other creative cross-linking methodologies. Although the term is used to refer to the "linking of polymer chains" for both sciences, the extent of crosslinking and specificities of the crosslinking agents vary greatly. As with all science, there are overlaps, and the following delineations are a starting point to understanding the subtleties. Polymer chemistry Crosslinking is the general term for the process ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyurethane
Polyurethane (; often abbreviated PUR and PU) refers to a class of polymers composed of organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethane is produced from a wide range of starting materials. This chemical variety produces polyurethanes with different chemical structures leading to many different applications. These include rigid and flexible foams, varnishes and coatings, adhesives, electrical potting compounds, and fibers such as spandex and PUL. Foams are the largest application accounting for 67% of all polyurethane produced in 2016. A polyurethane is typically produced by reacting an isocyanate with a polyol. Since a polyurethane contains two types of monomers, which polymerize one after the other, they are classed as alternating copolymers. Both the isocyanates and polyols used to make a polyurethane contain two or more functional groups per molecule. Global production in 2019 wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |