|
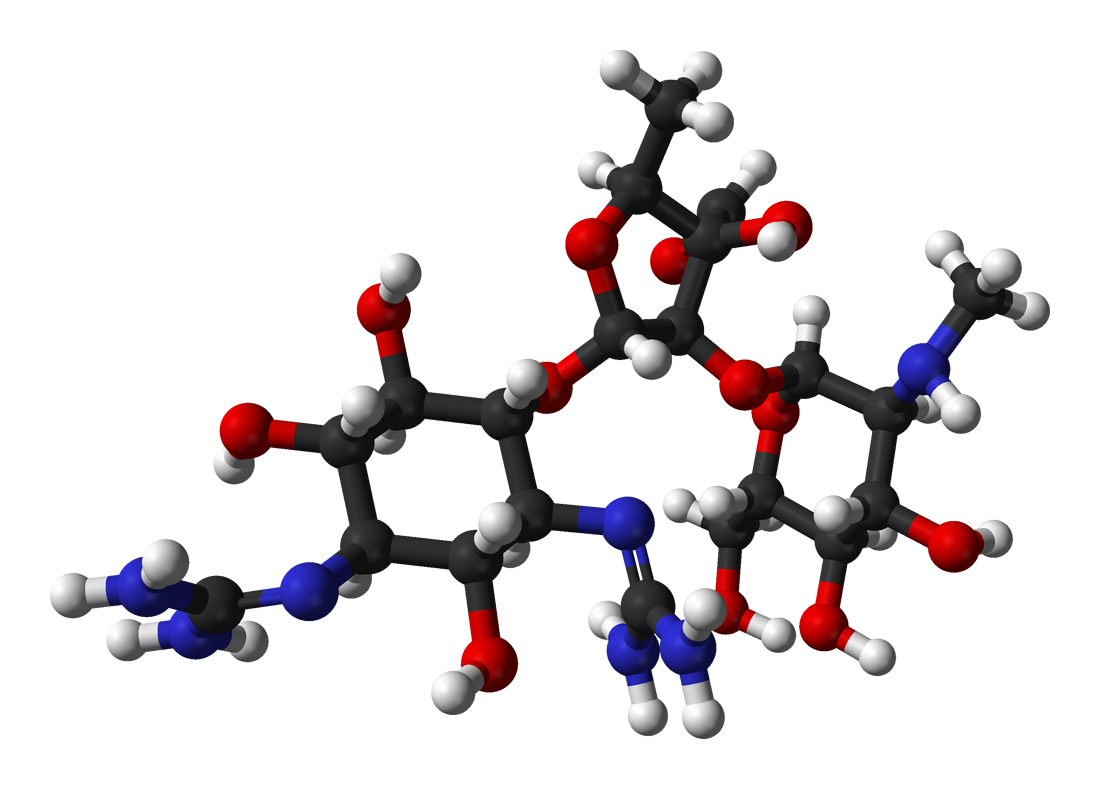
Hypothiocyanous Acid
Hypothiocyanite is the anion [OSCN]− and the conjugate base of hypothiocyanous acid (HOSCN). It is an organic compound part of the thiocyanates as it contains the functional group SCN. It is formed when an oxygen is singly bonded to the thiocyanate group. Hypothiocyanous acid is a fairly weak acid; its acid dissociation constant (p''K''a) is 5.3. Hypothiocyanite is formed by peroxidase catalysis of hydrogen peroxide and thiocyanate: : H2O2 + SCN− → OSCN− + H2O As a bactericide Hypothiocyanite occurs naturally in the antimicrobial immune system of the human respiratory tract in a redox reaction catalyzed by the enzyme lactoperoxidase. It has been researched extensively for its capabilities as an alternative antibiotic as it is harmless to human body cells while being cytotoxic to bacteria. The exact processes for making hypothiocyanite have been patented as such an effective antimicrobial has many commercial applications. Mechanism of action Lactoperoxidase-catalyse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons (e.g. K+ ( potassium ion)) while an anion is a negatively charged ion with more electrons than protons (e.g. Cl− ( chloride ion) and OH− ( hydroxide ion)). Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed ''monatomic ions'', ''atomic ions'' or ''simple ions'', while ions consisting of two or more atoms are termed polyatomic ions or ''molecular ions''. If only a + or − is present, it indi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bacillus Brevis
''Brevibacillus brevis'' (formerly known as ''Bacillus brevis'') is a Gram-positive, aerobic, motile, spore-forming, rod-shaped bacterium commonly found in soil, air, water, and decaying matter. It is rarely associated with infectious diseases. The antibiotics gramicidin and tyrocidine were first isolated from it.Abedon, Stephen. "Bacteria Binomials." 26 Apr 1998. Ohio State University. 17 Jun 2006 . ''Brevibacillus brevis'' is catalase positive, amylase negative, casein negative, gelatinase positive, and indole negative; most strains are citrate users. Some strains are capable of oxidizing carbon monoxide Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ... aerobically. Optimal growth occurs at 35 °C to 55 °C. References Paenibacillaceae Pathogenic bacteria B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Klebsiella Pneumoniae
''Klebsiella pneumoniae'' is a Gram-negative, non-motile, encapsulated, lactose- fermenting, facultative anaerobic, rod-shaped bacterium. It appears as a mucoid lactose fermenter on MacConkey agar. Although found in the normal flora of the mouth, skin, and intestines, it can cause destructive changes to human and animal lungs if aspirated, specifically to the alveoli, resulting in bloody, brownish or yellow colored jelly-like sputum. In the clinical setting, it is the most significant member of the genus ''Klebsiella'' of the Enterobacteriaceae. ''K. oxytoca'' and ''K. rhinoscleromatis'' have also been demonstrated in human clinical specimens. In recent years, ''Klebsiella'' species have become important pathogens in nosocomial infections. It naturally occurs in the soil, and about 30% of strains can fix nitrogen in anaerobic conditions. As a free-living diazotroph, its nitrogen-fixation system has been much-studied, and is of agricultural interest, as ''K. pneumoniae'' has ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Klebsiella Oxytoca
''Klebsiella oxytoca'' is a Gram-negative, rod-shaped bacterium that is closely related to '' K. pneumoniae'', from which it is distinguished by being indole-positive; it also has slightly different growth characteristics in that it is able to grow on melezitose, but not 3-hydroxybutyrate. It was first described in 1886 when it was isolated from sour milk and named ''Bacillus oxytocus perniciosus'' (from Greek ''oxus'' 'sour' + ''-tokos'' 'producing'). ''Klebsiella oxytoca'' is characterized by negative methyl red, positive VP, positive citrate, urea and TSI gas production, is AA, and negative for TSI sulfide, DNAse, growth on sulfide-indole motility medium and the phenylalanine deaminase test. It is a diazotroph, able to colonise plant hosts and fix atmospheric nitrogen into a form which the plant can use. Association of ''K. oxytoca'' with the barley rhizosphere during an entire vegetative period has been demonstrated. The bacteria adhere strongly to root hairs, and less stro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
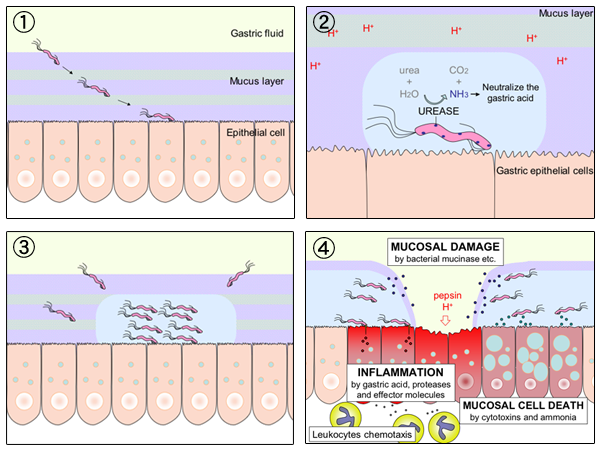
Helicobacter Pylori
''Helicobacter pylori'', previously known as ''Campylobacter pylori'', is a gram-negative, Flagellum#bacterial, flagellated, Bacterial cellular morphologies#Helical, helical bacterium. Mutants can have a rod or curved rod shape that exhibits less virulence. Its Helix, helical body (from which the genus name ''Helicobacter'' derives) is thought to have evolved to penetrate the gastric mucosa, mucous lining of the stomach, helped by its flagella, and thereby establish infection. While many earlier reports of an association between bacteria and the ulcers had existed, such as the works of John Lykoudis, it was only in 1983 when the bacterium was formally described for the first time in the English-language Western literature as the causal agent of peptic ulcer, gastric ulcers by Australian physician-scientists Barry Marshall and Robin Warren. In 2005, the pair was awarded the Nobel Prize in Physiology or Medicine for their discovery. Infection of the stomach with ''H. pylori'' doe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haemophilus Influenzae
''Haemophilus influenzae'' (formerly called Pfeiffer's bacillus or ''Bacillus influenzae'') is a Gram-negative, Motility, non-motile, Coccobacillus, coccobacillary, facultative anaerobic organism, facultatively anaerobic, Capnophile, capnophilic pathogenic bacterium of the family Pasteurellaceae. The bacteria are Mesophile, mesophilic and grow best at temperatures between 35 and 37 °C. ''H. influenzae'' was first described in 1893 by Richard Friedrich Johannes Pfeiffer, Richard Pfeiffer during an influenza pandemic when he incorrectly identified it as the causative microbe, which is why the bacteria was given the name "influenzae". ''H. influenzae'' is responsible for a wide range of localized and invasive infections, typically in infants and children, including pneumonia, meningitis, or bloodstream infections. Treatment consists of antibiotics; however, ''H. influenzae'' is often resistant to the penicillin family, but amoxicillin/clavulanic acid can be used in mild cases ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Escherichia Coli
''Escherichia coli'' ( )Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. is a gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus '' Escherichia'' that is commonly found in the lower intestine of warm-blooded organisms. Most ''E. coli'' strains are part of the normal microbiota of the gut, where they constitute about 0.1%, along with other facultative anaerobes. These bacteria are mostly harmless or even beneficial to humans. For example, some strains of ''E. coli'' benefit their hosts by producing vitamin K2 or by preventing the colonization of the intestine by harmful pathogenic bacteria. These mutually beneficial relationships between ''E. coli'' and humans are a type of mutualistic biological relationship—where both the humans and the ''E. coli'' are benefitting each other. ''E. coli'' is expelled into the environment within fecal matter. The bacterium grows massi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enterobacter Cloacae
''Enterobacter cloacae'' is a clinically significant Gram-negative, facultatively-anaerobic, rod-shaped bacterium. Microbiology In microbiology laboratories, ''E. cloacae'' is frequently grown at 30 °C on nutrient agar or at 35 °C in tryptic soy broth. It is a rod-shaped, Gram-negative Gram-negative bacteria are bacteria that, unlike gram-positive bacteria, do not retain the crystal violet stain used in the Gram staining method of bacterial differentiation. Their defining characteristic is that their cell envelope consists ... bacterium, is facultatively anaerobic, and bears peritrichous flagella. It is oxidase-negative and catalase-positive. Industrial use ''Enterobacter cloacae'' has been used in a bioreactor-based method for the biodegradation of explosives and in the biological control of plant diseases. ''Enterobacter cloacae'' strain MBB8 isolated from the Gulf of Mannar, India was reported to degrade poly vinyl alcohol (PVA). This was the f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corynebacterium Xerosis
''Corynebacterium xerosis'' is a gram-positive, rod-shaped bacterium in the genus ''Corynebacterium''. Although it is frequently a harmless Commensalism, commensal organism living on the skin and in the mucous membranes, ''C. xerosis'' is also a clinically relevant Opportunistic infection, opportunistic pathogen that has been attributed to many different infections in animals and humans. However, its actual prominence in human medicine is up for debate due to early difficulties distinguishing it from other ''Corynebacterium'' species in clinical isolates. Characteristics The genome of ''C. xerosis'' is approximately 2.7 million base pairs long with over 2,000 genes encoding proteins and a high GC-content, G+C content. C''. xerosis'' was found to contain a series of plasmids, one of which confers resistance to common antibiotics such as chloramphenicol, kanamycin, streptomycin, and tetracycline and was named pTP10. This plasmid has since been introduced into ''Bacillus subtilis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Capnocytophaga Ochracea
''Capnocytophaga'' is a genus of Gram-negative bacteria. Normally found in the oropharyngeal tract of mammals, they are involved in the pathogenesis of some animal bite wounds and periodontal diseases. Taxonomy The term ''Capnocytophaga'' comes from " capno-" for its dependence on CO2 and "cytophaga" for its flexibility and mobility shift (gliding motility). It belongs to the family Flavobacteriaceae, order Flavobacteriales. This genus includes eight different species: ''C. ochracea'', ''C. gingivalis'', ''C. granulosa'', ''C. haemolytica'', ''C. sputigena'', ''C. leadbetteri'' (isolated oral cavity of humans), ''C. canimorsus'', and ''C. cynodegmi'' (isolated from the oral cavity of animals). Many strains have also been described whose classification remains uncertain. Bacteriological isolation and identification ''Capnocytophaga'' spp. are fusiform Gram-negative bacilli, and they are part of the oral commensal flora. Microscopic observation revealed a high degree of polym ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Campylobacter Jejuni
''Campylobacter jejuni'' is a species of pathogenic bacteria that is commonly associated with poultry, and is also often found in animal feces. This species of microbe is one of the most common causes of food poisoning in Europe and in the US, with the vast majority of cases occurring as isolated events rather than mass outbreaks. Active surveillance through the Foodborne Diseases Active Surveillance Network (FoodNet) indicates that about 20 cases are ''diagnosed'' each year for each 100,000 people in the US, while many more cases are undiagnosed or unreported; the CDC estimates a total of 1.5 million infections every year. The European Food Safety Authority reported 246,571 cases in 2018, and estimated approximately nine million cases of human campylobacteriosis per year in the European Union. In Africa, Asia, and the Middle East, data indicates that ''C. jejuni'' infections are Endemic (epidemiology), endemic. ''Campylobacter'' is a genus of bacteria that is among the most ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Burkholderia Cepacia
''Burkholderia cepacia'' complex (BCC) is a species complex consisting of ''Burkholderia cepacia'' and at least 20 different biochemically similar species of Gram-negative bacteria. They are catalase-producing and lactose-nonfermenting. Members of BCC are opportunistic human pathogens that most often cause pneumonia in immunocompromised individuals with underlying lung disease (such as cystic fibrosis or chronic granulomatous disease). Patients with sickle-cell haemoglobinopathies are also at risk. The species complex also attacks young onion and tobacco plants, and displays a remarkable ability to digest oil. Taxonomy The group includes ''B. cepacia'', '' B. multivorans'', '' B. cenocepacia'', '' B. vietnamiensis'', '' B. stabilis'', '' B. ambifaria'', '' B. dolosa'', '' B. anthina'', '' B. pyrrocinia'' and '' B. ubonensis'', among other species. Occurrence BCC is resistant to a number of common disinfectants, specifi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |