|
Geranylgeranylation
Geranylgeranylation is a form of prenylation, which is a post-translational modification of proteins that involves the attachment of one or two 20-carbon lipophilic geranylgeranyl isoprene units from geranylgeranyl diphosphate to one or two cysteine residue(s) at the C-terminus of specific proteins. Prenylation (including geranylgeranylation) is thought to function, at least in part, as a membrane anchor for proteins. The process of geranylgeranylation can be catalyzed by either geranylgeranyl transferase I (GGTase I) or Rab GGTase (also GGTase II). GGTase I catalyzes the addition of one geranylgeranyl group onto the C-terminal consensus sequence CAAL (somewhat similar to farnesyltransferase reactions), where C=cysteine, A=any aliphatic amino acid, and L=leucine. Rab GGTase adds a total of two geranylgeranyl groups onto two cysteine residues at the C-terminal consensus sequence CXC or XXCC. The source of the geranylgeranyl group is geranylgeranyl diphosphate, which is synt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipid Anchor
Lipid-anchored proteins (also known as lipid-linked proteins) are proteins located on the surface of the cell membrane that are covalently attached to lipids embedded within the cell membrane. These proteins insert and assume a place in the bilayer structure of the membrane alongside the similar fatty acid tails. The lipid-anchored protein can be located on either side of the cell membrane. Thus, the lipid serves to anchor the protein to the cell membrane. They are a type of proteolipids. The lipid groups play a role in protein interaction and can contribute to the function of the protein to which it is attached. Furthermore, the lipid serves as a mediator of membrane associations or as a determinant for specific protein-protein interactions. For example, lipid groups can play an important role in increasing molecular hydrophobicity. This allows for the interaction of proteins with cellular membranes and protein domains. In a dynamic role, lipidation can sequester a protein away ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prenylation
Prenylation (also known as isoprenylation or lipidation) is the addition of hydrophobic molecules to a protein or a biomolecule. It is usually assumed that prenyl groups (3-methylbut-2-en-1-yl) facilitate attachment to cell membranes, similar to lipid anchors like the GPI anchor, though direct evidence of this has not been observed. Prenyl groups (also called isoprenyl groups, having one hydrogen atom more than isoprene) have been shown to be important for protein–protein binding through specialized prenyl-binding domains. Protein prenylation Protein prenylation involves the transfer of either a farnesyl or a geranylgeranyl moiety to C-terminal cysteine(s) of the target protein. There are three enzymes that carry out prenylation in the cell, farnesyl transferase, Caax protease and geranylgeranyl transferase I. Farnesylation is a type of prenylation, a post-translational modification of proteins by which an isoprenyl group is added to a cysteine residue. It is an important p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoprenoid
The terpenoids, also known as isoprenoids, are a class of naturally occurring organic chemicals derived from the 5-carbon compound isoprene and its derivatives called terpenes, diterpenes, etc. While sometimes used interchangeably with "terpenes", terpenoids contain additional functional groups, usually containing oxygen. When combined with the hydrocarbon terpenes, terpenoids comprise about 80,000 compounds. They are the largest class of plant secondary metabolites, representing about 60% of known natural products. Many terpenoids have substantial pharmacological bioactivity and are therefore of interest to medicinal chemists. Plant terpenoids are used for their aromatic qualities and play a role in traditional herbal remedies. Terpenoids contribute to the scent of eucalyptus, the flavors of cinnamon, cloves, and ginger, the yellow color in sunflowers, and the red color in tomatoes. Well-known terpenoids include citral, menthol, camphor, salvinorin A in the plant '' Salvi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteins
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
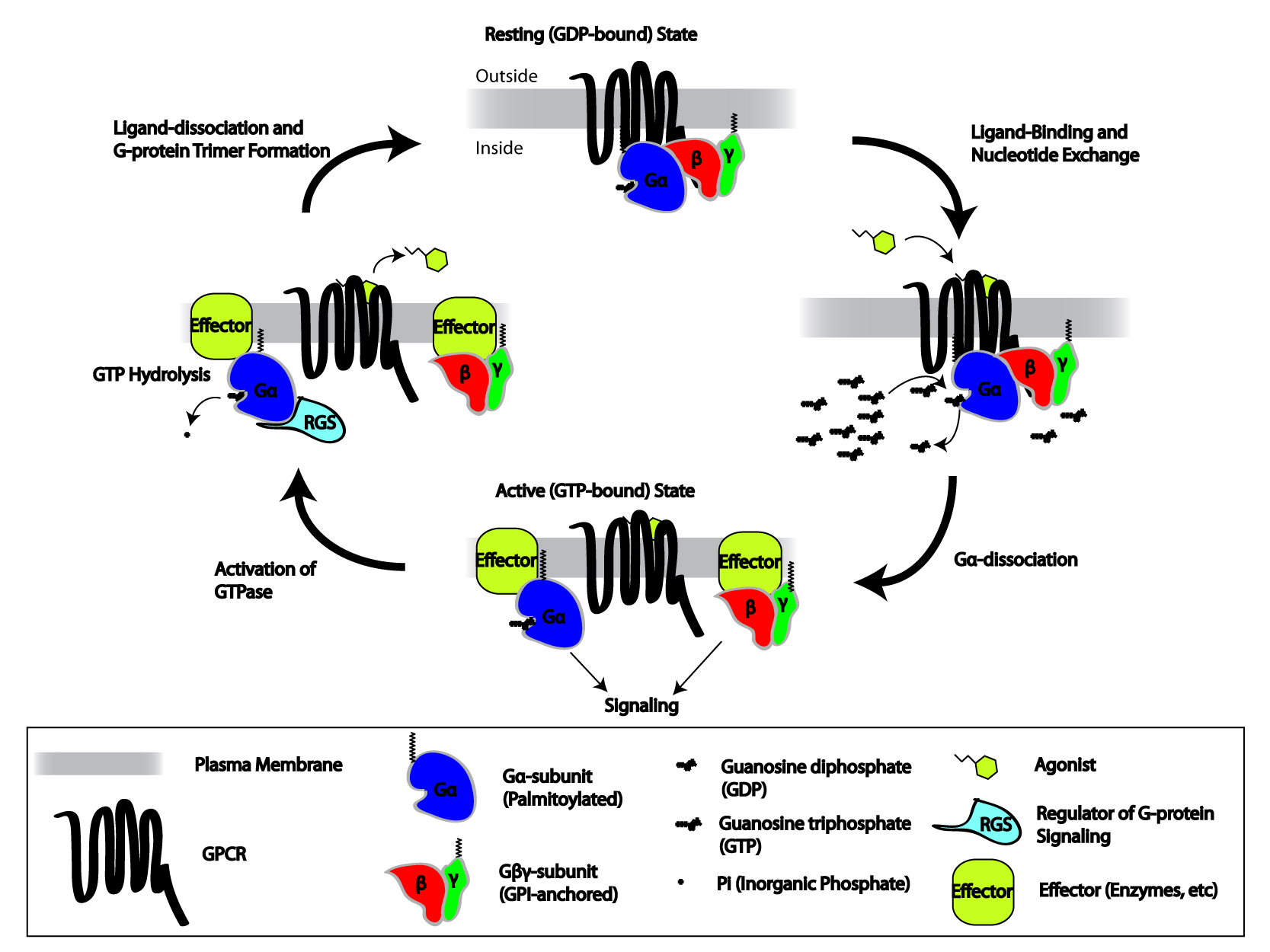
Heterotrimeric G Proteins
Heterotrimeric G protein, also sometimes referred to as the ''"large" G proteins'' (as opposed to the subclass of smaller, monomeric small GTPases) are membrane-associated G proteins that form a heterotrimeric complex. The biggest non-structural difference between heterotrimeric and monomeric G protein is that heterotrimeric proteins bind to their cell-surface receptors, called G protein-coupled receptors, directly. These G proteins are made up of ''alpha'' (α), ''beta'' (β) and ''gamma'' (γ) subunits. The alpha subunit is attached to either a GTP or GDP, which serves as an on-off switch for the activation of G-protein. When ligands bind a GPCR, the GPCR acquires GEF (guanine nucleotide exchange factor) ability, which activates the G-protein by exchanging the GDP on the ''alpha'' subunit to GTP. The binding of GTP to the ''alpha'' subunit results in a structural change and its dissociation from the rest of the G-protein. Generally, the ''alpha'' subunit binds membrane-bound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
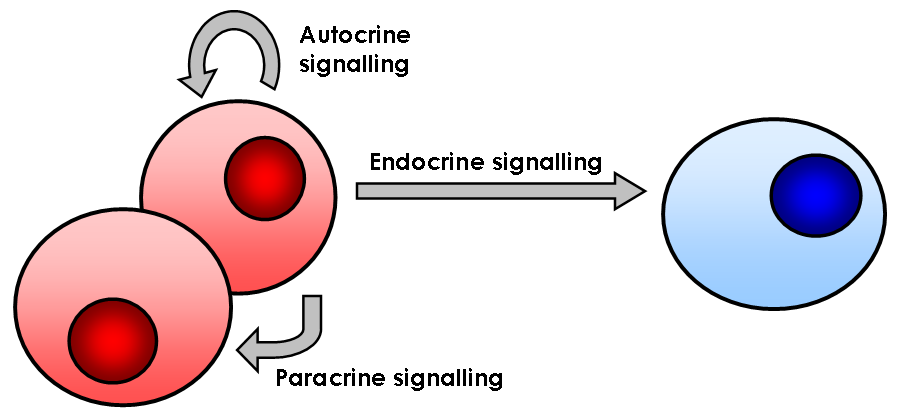
Signaling Molecule
In biology, cell signaling (cell signalling in British English) or cell communication is the ability of a Cell (biology), cell to receive, process, and transmit signals with its environment and with itself. Cell signaling is a fundamental property of all Cell (biology), cellular life in prokaryotes and eukaryotes. Signals that originate from outside a cell (or extracellular signals) can be physical agents like mechanical pressure, membrane potential , voltage, temperature, light, or chemical signals (e.g., small molecules, peptides, or gas). Cell signaling can occur over short or long distances, and as a result can be classified as autocrine, juxtacrine, intracrine, paracrine, or endocrine. Signaling molecules can be synthesized from various biosynthetic pathways and released through passive transport , passive or active transports, or even from cell damage. Receptor (biochemistry), Receptors play a key role in cell signaling as they are able to detect chemical signals or physic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rho Family Of GTPases
The Rho family of GTPases is a family of small (~21 kDa) signaling G proteins, and is a subfamily of the Ras superfamily. The members of the Rho GTPase family have been shown to regulate many aspects of intracellular actin dynamics, and are found in all eukaryotic kingdoms, including yeasts and some plants. Three members of the family have been studied in detail: Cdc42, Rac1, and RhoA. All G proteins are "molecular switches", and Rho proteins play a role in organelle development, cytoskeletal dynamics, cell movement, and other common cellular functions. History Identification of the Rho family of GTPases began in the mid-1980s. The first identified Rho member was RhoA, isolated serendipitously in 1985 from a low stringency cDNA screening. Rac1 and Rac2 were identified next, in 1989 followed by Cdc42 in 1990. Eight additional mammalian Rho members were identified from biological screenings until the late 1990s, a turning point in biology where availability of complete genome seq ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anti-Cancer Agents In Medicinal Chemistry
''Anti-Cancer Agents in Medicinal Chemistry'' is a peer-reviewed academic journal covering the disciplines of medicinal chemistry and drug design relating to chemotherapeutic agents in cancer. It is published by Bentham Science Publishers and the editor-in-chief is Simone Carradori ( "G. d'Annunzio" University of Chieti-Pescara). The journal covers developments in "medicinal chemistry and rational drug design for the discovery of anti-cancer agents" and publishes original research reports and review papers. It is related to the journal ''Current Medicinal Chemistry'' and was established in 2001 as ''Current Medicinal Chemistry – Anti-Cancer Agents''. The journal obtained its present title in 2006. Abstracting and indexing The journal is abstracted and indexed in: According to the ''Journal Citation Reports'', the journal has a 2020 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GGPS1
Geranylgeranyl pyrophosphate synthase is an enzyme that in humans is encoded by the ''GGPS1'' gene. Function This gene is a member of the prenyltransferase family and encodes a protein with geranylgeranyl diphosphate (GGPP) synthase activity. The enzyme catalyzes the synthesis of GGPP from farnesyl diphosphate and isopentenyl diphosphate. GGPP is an important molecule responsible for the C20-prenylation of proteins and for the regulation of a nuclear hormone receptor. Alternate transcriptional splice variants, encoding different isoforms, have been characterized. Much like its homolog farnesyl diphosphate synthase, GGPS1 is inhibited by bisphosphonate Bisphosphonates are a class of drugs that prevent the loss of bone density, used to treat osteoporosis and similar diseases. They are the most commonly prescribed drugs used to treat osteoporosis. They are called bisphosphonates because they ... compounds. Clinical Mutations in both copies of this gene have been associ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Post-translational Modification
Post-translational modification (PTM) is the covalent and generally enzymatic modification of proteins following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus. Proteins are synthesized by ribosomes translating mRNA into polypeptide chains, which may then undergo PTM to form the mature protein product. PTMs are important components in cell signaling, as for example when prohormones are converted to hormones. Post-translational modifications can occur on the amino acid side chains or at the protein's C- or N- termini. They can extend the chemical repertoire of the 20 standard amino acids by modifying an existing functional group or introducing a new one such as phosphate. Phosphorylation is a highly effective mechanism for regulating the activity of enzymes and is the most common post-translational modification. Many eukaryotic and prokaryotic proteins also have carbohydrate molecules attached to them in a process called gly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rab Geranylgeranyltransferase
Rab geranylgeranyltransferase also known as (protein) geranylgeranyltransferase II is one of the three prenyltransferases. It transfers (usually) two geranylgeranyl groups to the cystein(s) at the C-terminus of Rab proteins. :geranylgeranyl diphosphate + protein-cysteine \rightleftharpoons S-geranylgeranyl-Cys-protein + diphosphate The C-terminus of Rab proteins varies in length and sequence and is referred to as hypervariable. Thus Rab proteins do not have a consensus sequence, such as the CAAX box, which the Rab geranylgeranyltransferase can recognise. Instead Rab proteins are bound by the Rab escort protein (REP) over a more conserved region of the Rab protein and then presented to the Rab geranylgeranyltransferase. Once Rab proteins are prenylated, the lipid anchor(s) ensure that Rabs are no longer soluble. REP therefore plays an important role in binding and solubilising the geranylgeranyl groups and delivers the Rab protein to the relevant cell membrane. Reaction Rab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geranylgeranyltransferase Type 1
Geranylgeranyltransferase type 1 or simply geranylgeranyltransferase is one of the three enzymes in the prenyltransferase group. In specific terms, Geranylgeranyltransferase (GGTase 1) adds a 20-carbon isoprenoid called a geranylgeranyl group to proteins bearing a CaaX motif: a four-amino acid sequence at the carboxyl terminal of a protein. Geranylgeranyltransferase inhibitors are being investigated as anti-cancer agents. Function Prenyltransferases, including geranylgeranyltransferase, posttranslationally modify proteins by adding an isoprenoid lipid called a prenyl group to the carboxyl terminus of the target protein. This process, called prenylation, causes prenylated proteins to become membrane-associated due to the hydrophobic nature of the prenyl group. Most prenylated proteins are involved in cellular signaling, wherein membrane association is critical for function. Structure Geranylgeranyltransferase contains two subunits, α and β that are encoded by the ''FNTA ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |