|
Fusion Power
Fusion power is a proposed form of power generation that would generate electricity by using heat from nuclear fusion reactions. In a fusion process, two lighter atomic nuclei combine to form a heavier nucleus, while releasing energy. Devices designed to harness this energy are known as fusion reactors. Research into fusion reactors began in the 1940s, but as of 2025, no device has reached net power. Fusion processes require fuel, in a state of plasma, and a confined environment with sufficient temperature, pressure, and confinement time. The combination of these parameters that results in a power-producing system is known as the Lawson criterion. In stellar cores the most common fuel is the lightest isotope of hydrogen (Protium (isotope), protium), and gravity provides the conditions needed for fusion energy production. Proposed fusion reactors would use the heavy hydrogen isotopes of deuterium and tritium for DT fusion, for which the Lawson criterion is the easiest to achieve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scylla I
In Greek mythology, Scylla ( ; , ) is a legendary, man-eating monster that lives on one side of a narrow channel of water, opposite her counterpart, the sea-swallowing monster Charybdis. The two sides of the strait are within an arrow's range of each other—so close that sailors attempting to avoid the whirlpools of Charybdis would pass dangerously close to Scylla and vice versa. Scylla is first attested in Homer's ''Odyssey'', where Odysseus and his crew encounter her and Charybdis on their travels. Later myth provides an origin story as a beautiful nymph who gets turned into a monster. Book Three of Virgil's ''Aeneid'' associates the strait where Scylla dwells with the Strait of Messina between Calabria, a region of Southern Italy, and Sicily. The coastal town of Scilla, Calabria, Scilla in Calabria takes its name from the mythological figure of Scylla and it is said to be the home of the nymph. The idiom "between Scylla and Charybdis" has come to mean being forced to choose ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
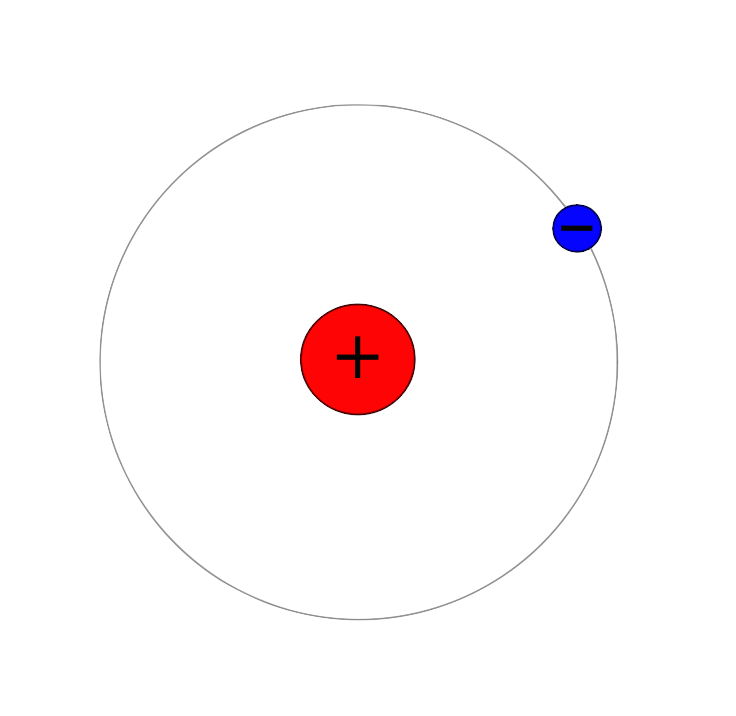
Atomic Nuclei
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford at the University of Manchester based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. An atom is composed of a positively charged nucleus, with a cloud of negatively charged electrons surrounding it, bound together by electrostatic force. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force. The diameter of the nucleus is in the range of () for hydrogen (the diameter of a single proton) to about for uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fusion Energy Gain Factor
A fusion energy gain factor, usually expressed with the symbol ''Q'', is the ratio of fusion power produced in a nuclear fusion reactor to the power required to maintain the plasma in steady state. The condition of ''Q'' = 1, when the power being released by the fusion reactions is equal to the required heating power, is referred to as breakeven, or in some sources, scientific breakeven. The energy given off by the fusion reactions may be captured within the fuel, leading to ''self-heating''. Most fusion reactions release at least some of their energy in a form that cannot be captured within the plasma, so a system at ''Q'' = 1 will cool without external heating. With typical fuels, self-heating in fusion reactors is not expected to match the external sources until at least ''Q'' ≈ 5. If ''Q'' increases past this point, increasing self-heating eventually removes the need for external heating. At this point the reaction becomes self-sustaining, a c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nuclear fission in 1938, the first self-sustaining nuclear reactor (Chicago Pile-1, 1942) and the first nuclear weapon (Trinity (nuclear test), Trinity, 1945). Neutrons are found, together with a similar number of protons in the atomic nucleus, nuclei of atoms. Atoms of a chemical element that differ only in neutron number are called isotopes. Free neutrons are produced copiously in nuclear fission and nuclear fusion, fusion. They are a primary contributor to the nucleosynthesis of chemical elements within stars through fission, fusion, and neutron capture processes. Neutron stars, formed from massive collapsing stars, consist of neutrons at the density of atomic nuclei but a total mass more than the Sun. Neutron properties and interactions ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is the lowest among all the Chemical element, elements, and it does not have a melting point at standard pressures. It is the second-lightest and second-most Abundance of the chemical elements, abundant element in the observable universe, after hydrogen. It is present at about 24% of the total elemental mass, which is more than 12 times the mass of all the heavier elements combined. Its abundance is similar to this in both the Sun and Jupiter, because of the very high nuclear binding energy (per nucleon) of helium-4 with respect to the next three elements after helium. This helium-4 binding energy also accounts for why it is a product of both nuclear fusion and radioactive decay. The most common isotope of helium in the universe is helium-4, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DT Fusion
DT may refer to: Arts Music * "D.T.", an instrumental song on ''Who Made Who'', AC/DC's 1986 album * Dark Tranquillity, Swedish melodic death metal band * Dream Theater, American progressive metal band * MC DT, a UK garage emcee and member of DJ Pied Piper and the Masters of Ceremonies Other media * The Dark Tower (other), various works of fiction * Dilithium (''Star Trek''), fictional chemical element by its symbol * '' Ixion Saga DT'', a television series * An abbreviation for documentary theatre Businesses and organisations * Daimler Truck, German commercial vehicle manufacturer * Dalarnas Tidningar, Swedish newspaper and media company * Deutsche Telekom (by NYSE ticker symbol) * Dhanmondi Tutorial, an educational organisation * Dimosia Tileorasi, a former Greek public broadcaster * DT Infrastructure, Australian construction company * Dynatrace, software intelligence provider (by NYSE stock symbol) * TAAG Angola Airlines (IATA code: DT) * Turkish State Theat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tritium
Tritium () or hydrogen-3 (symbol T or H) is a rare and radioactive isotope of hydrogen with a half-life of ~12.33 years. The tritium nucleus (t, sometimes called a ''triton'') contains one proton and two neutrons, whereas the nucleus of the common isotope hydrogen-1 (''protium'') contains one proton and no neutrons, and that of non-radioactive hydrogen-2 ('' deuterium'') contains one proton and one neutron. Tritium is the heaviest particle-bound isotope of hydrogen. It is one of the few nuclides with a distinct name. The use of the name hydrogen-3, though more systematic, is much less common. Naturally occurring tritium is extremely rare on Earth. The atmosphere has only trace amounts, formed by the interaction of its gases with cosmic rays. It can be produced artificially by irradiation of lithium or lithium-bearing ceramic pebbles in a nuclear reactor and is a low-abundance byproduct in normal operations of nuclear reactors. Tritium is used as the energy source in radio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deuterium
Deuterium (hydrogen-2, symbol H or D, also known as heavy hydrogen) is one of two stable isotopes of hydrogen; the other is protium, or hydrogen-1, H. The deuterium nucleus (deuteron) contains one proton and one neutron, whereas the far more common H has no neutrons. The name ''deuterium'' comes from Greek '' deuteros'', meaning "second". American chemist Harold Urey discovered deuterium in 1931. Urey and others produced samples of heavy water in which the H had been highly concentrated. The discovery of deuterium won Urey a Nobel Prize in 1934. Nearly all deuterium found in nature was synthesized in the Big Bang 13.8 billion years ago, forming the primordial ratio of H to H (~26 deuterium nuclei per 10 hydrogen nuclei). Deuterium is subsequently produced by the slow stellar proton–proton chain, but rapidly destroyed by exothermic fusion reactions. The deuterium–deuterium reaction has the second-lowest energy threshold, and is the most astrophysically acce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Isotope
Hydrogen (H) has three naturally occurring isotopes: H, H, and H. H and H are stable, while H has a half-life of years. Heavier isotopes also exist; all are synthetic and have a half-life of less than 1 zeptosecond (10 s). Of these, H is the least stable, while H is the most. Hydrogen is the only chemical element, element whose isotopes have different names that remain in common use today: H is deuterium and H is tritium. The symbols D and T are sometimes used for deuterium and tritium; IUPAC (International Union of Pure and Applied Chemistry) accepts said symbols, but recommends the standard isotopic symbols H and H, to avoid confusion in alphabetic sorting of chemical formulas. H, with no neutrons, may be called protium to disambiguate. (During the early study of radioactivity, some other heavy radioisotopes were given Chemical symbol#Symbols for named isotopes, names, but such names are rarely used today.) List of isotopes Note: "y" means year, but "ys" means yoctose ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gravity
In physics, gravity (), also known as gravitation or a gravitational interaction, is a fundamental interaction, a mutual attraction between all massive particles. On Earth, gravity takes a slightly different meaning: the observed force between objects and the Earth. This force is dominated by the combined gravitational interactions of particles but also includes effect of the Earth's rotation. Gravity gives weight to physical objects and is essential to understanding the mechanisms responsible for surface water waves and lunar tides. Gravity also has many important biological functions, helping to guide the growth of plants through the process of gravitropism and influencing the circulation of fluids in multicellular organisms. The gravitational attraction between primordial hydrogen and clumps of dark matter in the early universe caused the hydrogen gas to coalesce, eventually condensing and fusing to form stars. At larger scales this results in galaxies and clust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protium (isotope)
Hydrogen (H) has three naturally occurring isotopes: H, H, and H. H and H are stable, while H has a half-life of years. Heavier isotopes also exist; all are synthetic and have a half-life of less than 1 zeptosecond (10 s). Of these, H is the least stable, while H is the most. Hydrogen is the only element whose isotopes have different names that remain in common use today: H is deuterium and H is tritium. The symbols D and T are sometimes used for deuterium and tritium; IUPAC (International Union of Pure and Applied Chemistry) accepts said symbols, but recommends the standard isotopic symbols H and H, to avoid confusion in alphabetic sorting of chemical formulas. H, with no neutrons, may be called protium to disambiguate. (During the early study of radioactivity, some other heavy radioisotopes were given names, but such names are rarely used today.) List of isotopes Note: "y" means year, but "ys" means yoctosecond (10 second). , - , H , 1 , 0 , , colspan=3 alig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the chemical formula, formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |