|
Fluoroform
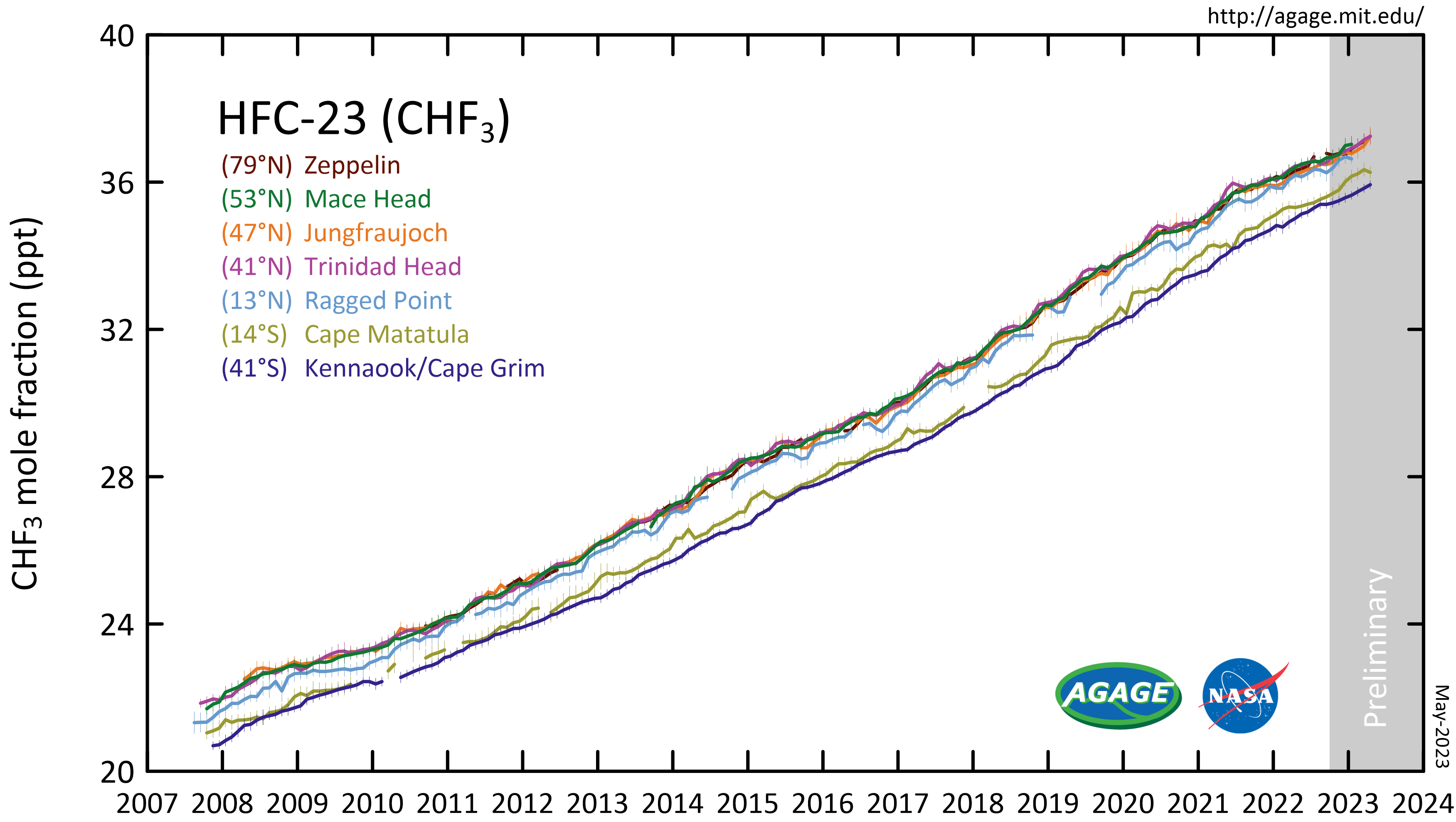
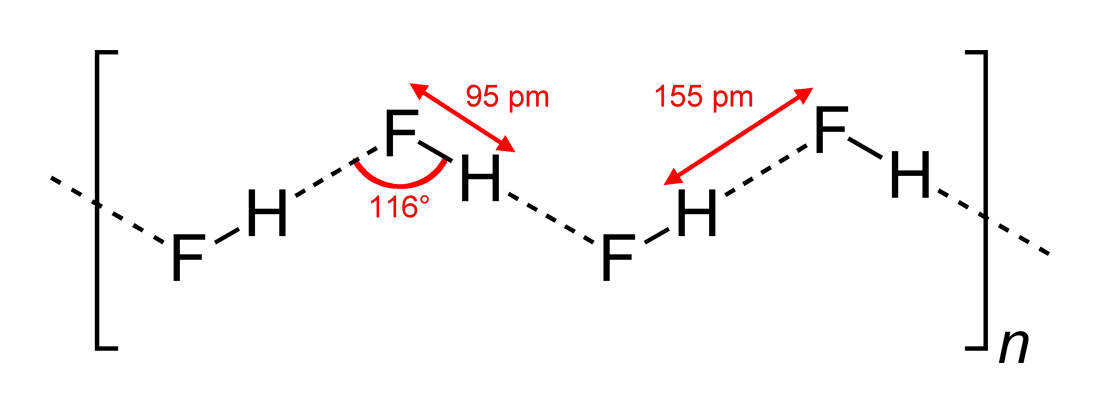
Fluoroform, or trifluoromethane, is the chemical compound with the formula . It is a hydrofluorocarbon as well as being a part of the haloforms, a class of compounds with the formula (X = halogen) with C3v symmetry. Fluoroform is used in diverse applications in organic synthesis. It is not an ozone depleter but is a greenhouse gas. Synthesis About 20 million kg per year are produced industrially as both a by-product of and precursor to the manufacture of Teflon. It is produced by reaction of chloroform with HF: : It is also generated biologically in small amounts apparently by decarboxylation of trifluoroacetic acid. Historical Fluoroform was first obtained by Maurice Meslans in the violent reaction of iodoform with dry silver fluoride in 1894. The reaction was improved by Otto Ruff by substitution of silver fluoride by a mixture of mercury fluoride and calcium fluoride. The exchange reaction works with iodoform and bromoform, and the exchange of the first two haloge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trihalomethane
In chemistry, trihalomethanes (THMs) are chemical compounds in which three of the four hydrogen atoms of methane () are replaced by halogen atoms. Trihalomethanes with all the same halogen atoms are called haloforms. Many trihalomethanes find uses in industry as solvents or refrigerants. Some THMs are also environmental pollutants, and a few are considered carcinogenic. Table of common trihalomethanes Industrial uses Only chloroform has significant applications of the haloforms. In the predominant application, chloroform is required for the production of tetrafluoroethylene (TFE), precursor to teflon. Chloroform is fluorinated by reaction with hydrogen fluoride to produce chlorodifluoromethane (R-22). Pyrolysis of chlorodifluoromethane (at 550-750 °C) yields TFE, with difluorocarbene as an intermediate. :CHCl3 + 2 HF -> CHClF2 + 2 HCl :2 CHClF2 -> C2F4 + 2 HCl Refrigerants and solvents Trihalomethanes released to the environment break down faster than chlorofluorocarbons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trifluoroacetic Acid
Trifluoroacetic acid (TFA) is a synthetic organofluorine compound with the chemical formula CF3CO2H. It belongs to the subclass of per- and polyfluoroalkyl substances (PFASs) known as ultrashort-chain perfluoroalkyl acids (PFAAs). TFA is not produced biologically or abiotically and is commonly used in organic chemistry for various purposes. It is the most abundant PFAS found in the environment. It is a haloacetic acid, with all three of the acetyl group's hydrogen atoms replaced by fluorine atoms. It is a colorless liquid with a vinegar-like odor. TFA is a stronger acid than acetic acid, having an acid ionisation constant, ''K''a, that is approximately 34,000 times higher, as the highly electronegative fluorine atoms and consequent electron-withdrawing nature of the trifluoromethyl group weakens the oxygen-hydrogen bond (allowing for greater acidity) and stabilises the anionic conjugate base. Synthesis TFA is prepared industrially by the electrofluorination of ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromoform
Bromoform is an organic compound with the chemical formula . It is a colorless liquid at room temperature, with a high refractive index and a very high density. Its sweet odor is similar to that of chloroform. It is one of the four haloforms, the others being fluoroform, chloroform, and iodoform. It is a brominated organic solvent. Currently its main use is as a laboratory reagent. It is very slightly soluble in water (one part bromoform in 800 parts water) and is miscible with alcohol, benzene, chloroform, ether, petroleum ether, acetone and oils. Structure The molecule adopts tetrahedral molecular geometry with C3v symmetry. Synthesis Bromoform was discovered in 1832 by Löwig who distilled a mixture of bromal and potassium hydroxide, as analogous to preparation of chloroform from chloral. Bromoform can be prepared by the haloform reaction using acetone and sodium hypobromite, by the electrolysis of potassium bromide in ethanol, or by treating chloroform with aluminium brom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver Fluoride
Silver fluoride can refer to: * Silver subfluoride (disilver monofluoride), Ag2F * Silver(I) fluoride (silver monofluoride, argentous fluoride), AgF * Silver(I,II) fluorides (disilver trifluoride, trisilver tetrafluoride) Ag2F3, Ag3F4 * Silver(II) fluoride (silver difluoride, argentic fluoride), AgF2 * Silver(II,III) fluorides (disilver pentafluoride, trisilver octafluoride) Ag2F5, Ag3F8 * Silver(III) fluoride (silver trifluoride), AgF3 * Silver diammine fluoride, a material used to stop dental caries Tooth decay, also known as caries,The word 'caries' is a mass noun, and is not a plural of 'carie'.'' is the breakdown of teeth due to acids produced by bacteria. The resulting cavities may be a number of different colors, from yellow to black ... (cavities), AgFH6N2 Gallery References {{Reflist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodoform
Iodoform (also known as triiodomethane) is the organoiodine compound with the chemical formula . It is a pale yellow, crystalline, volatile substance, with a penetrating and distinctive odor (in older chemistry texts, the smell is sometimes referred to as that of hospitals, where the compound is still commonly used) and, analogous to chloroform, sweetish taste. It is occasionally used as a disinfectant. Naming The name iodoform originates with the "formyle radical," an archaic term for the HC moiety, and is retained for historical consistency. A full, modern name is triiodomethane. Another possible name is "carbon hydride triiodide". The "hydride" in the latter is sometimes omitted, though the IUPAC recommends against doing so, as "carbon triiodide" could also mean (hexaiodoethane, a highly unstable compound). Structure The molecule adopts a tetrahedral molecular geometry, tetrahedral geometry with C3v symmetry group, symmetry. Synthesis and reactions The synthesis of iodofo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maurice Meslans
Maurice Meslans (1862–1938) was a French pharmacist and chemist, Henri Moissan Ferdinand Frédéric Henri Moissan (; 28 September 1852 – 20 February 1907) was a French chemist and pharmacist who won the 1906 Nobel Prize in Chemistry for his work in isolating fluorine from its compounds. Among his other contributions, Mo ...'s advanced student, and a pioneer in organofluorocompounds chemistry. References 1862 births 1938 deaths 20th-century French chemists Nancy-Université alumni 19th-century French chemists {{france-chemist-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carboxylation, the addition of CO2 to a compound. Enzymes that catalyze decarboxylations are called decarboxylases or, the more formal term, carboxy-lyases (Enzyme Commission number, EC number 4.1.1). In organic chemistry The term "decarboxylation" usually means replacement of a carboxyl group () with a hydrogen atom: : Decarboxylation is one of the oldest known organic reactions. It is one of the processes assumed to accompany pyrolysis and destructive distillation. Overall, decarboxylation depends upon stability of the carbanion synthon , although the anion may not be a true chemical intermediate. Typically, carboxylic acids decarboxylate slowly, but carboxylic acids with an α el ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroform
Chloroform, or trichloromethane (often abbreviated as TCM), is an organochloride with the formula and a common solvent. It is a volatile, colorless, sweet-smelling, dense liquid produced on a large scale as a precursor to refrigerants and polytetrafluoroethylene (PTFE). Chloroform was once used as an inhalational anesthetic between the 19th century and the first half of the 20th century. It is miscible with many solvents but it is only very slightly soluble in water (only 8 g/L at 20°C). Structure and name The molecule adopts a tetrahedral molecular geometry with C3v symmetry. The chloroform molecule can be viewed as a methane molecule with three hydrogen atoms replaced with three chlorine atoms, leaving a single hydrogen atom. The name "chloroform" is a portmanteau of ''terchloride'' (tertiary chloride, a trichloride) and ''formyle'', an obsolete name for the methylylidene radical (CH) derived from formic acid. Natural occurrence Many kinds of seaweed produce chlor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Teflon
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene, and has numerous applications because it is chemically inert. The commonly known brand name of PTFE-based composition is Teflon by Chemours, a spin-off from DuPont, which originally invented the compound in 1938. Polytetrafluoroethylene is a fluorocarbon solid, as it is a high- molecular-weight polymer consisting wholly of carbon and fluorine. PTFE is hydrophobic: neither water nor water-containing substances wet PTFE, as fluorocarbons exhibit only small London dispersion forces due to the low electric polarizability of fluorine. PTFE has one of the lowest coefficients of friction of any solid. Polytetrafluoroethylene is used as a non-stick coating for pans and other cookware. It is non-reactive, partly because of the strength of carbon–fluorine bonds, so it is often used in containers and pipework for reactive and corrosive chemicals. When used as a lubricant, PTFE reduces fric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Greenhouse Gas
Greenhouse gases (GHGs) are the gases in the atmosphere that raise the surface temperature of planets such as the Earth. Unlike other gases, greenhouse gases absorb the radiations that a planet emits, resulting in the greenhouse effect. The Earth is warmed by sunlight, causing its surface to radiate heat, which is then mostly absorbed by greenhouse gases. Without greenhouse gases in the atmosphere, the average temperature of Earth's surface would be about , rather than the present average of .Le Treut, H., R. Somerville, U. Cubasch, Y. Ding, C. Mauritzen, A. Mokssit, T. Peterson and M. Prather, 2007:Chapter 1: Historical Overview of Climate Change. In:Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. olomon, S., D. Qin, M. Manning, Z. Chen, M. Marquis, K.B. Averyt, M. Tignor and H.L. Miller (eds.) Cambridge University Press, Cambridge, United Kingdom and New Y ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |