|
Ethane-1,2-dithiol
Ethane-1,2-dithiol, also known as EDT, is a colorless liquid with the Chemical formula, formula Carbon, CHydrogen, H(Thiol, SH). It has a very characteristic odor which is compared by many people to rotten cabbage. It is a common building block in organic synthesis and an excellent ligand for metal ions. Preparation Ethane-1,2-dithiol is made commercially by the reaction of 1,2-dichloroethane with aqueous sodium bisulfide. In the laboratory, it can also be prepared by the action of 1,2-dibromoethane on thiourea followed by hydrolysis. Applications As a 1,2-dithiol, this compound is widely used in organic chemistry because it reacts with aldehydes and ketones to give 1,3-dithiolanes, which are useful intermediates. R. E. Conrow "Ethanedithiol" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. ::CH(SH) + RR'CO → CHSCRR' + HO Other 1,2- and 1,3-dithiols undergo this reaction to give related 1,3-dithiolanes and 1,3-dithianes ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,1-Ethanedithiol
Ethane-1,1-dithiol is an organosulfur compound with formula CH3CH(SH)2. It is a colourless smelly liquid that is added to or found in some foods. The compound is an example of a geminal dithiol. Use Flavouring uses of ethane-1,1-dithiol may include drinks, oil, gravy, soup, meat, fruit, seasonings, and snacks. Maximum concentrations in use that are generally recognised as safe (GRAS) is five parts per million (ppm) but typical uses are around 0.2 ppm. It is supplied as a 1% solution in ethanol, due to its strong offensive smell. In the diluted form with 4% ethyl acetate and ethanol the CAS number is 69382-62-3. Toxicity may be due to metabolism products hydrogen sulfide and acetaldehyde, however as used it has a margin of safety of over 10,000,000. Other ways that it is modified in the body apart from hydrolysis is methylation to 1-methylsulfanyl-ethanethiol, oxidation of the sulfur to an ethyl sulfonate, glucuronidation of the sulfur, or combination with cysteine by way of a di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethane-1,1-dithiol
Ethane-1,1-dithiol is an organosulfur compound with formula CH3CH(SH)2. It is a colourless smelly liquid that is added to or found in some foods. The compound is an example of a geminal dithiol. Use Flavouring uses of ethane-1,1-dithiol may include drinks, oil, gravy, soup, meat, fruit, seasonings, and snacks. Maximum concentrations in use that are generally recognised as safe (GRAS) is five parts per million (ppm) but typical uses are around 0.2 ppm. It is supplied as a 1% solution in ethanol, due to its strong offensive smell. In the diluted form with 4% ethyl acetate and ethanol the CAS number is 69382-62-3. Toxicity may be due to metabolism products hydrogen sulfide and acetaldehyde, however as used it has a margin of safety of over 10,000,000. Other ways that it is modified in the body apart from hydrolysis is methylation to 1-methylsulfanyl-ethanethiol, oxidation of the sulfur to an ethyl sulfonate, glucuronidation of the sulfur, or combination with cysteine by way of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dithiolane
A dithiolane is a sulfur heterocyclic compound, heterocycle derived from cyclopentane by replacing two methylene bridges (-- units) with thioether groups. The parent compounds are 1,2-dithiolane and 1,3-dithiolane. 1,2-Dithiolanes are cyclic disulfides. Some dithiolanes are natural products that can be found in foods, such as asparagusic acid in asparagus. The 4-dimethylamino derivative nereistoxin was the inspiration for insecticides which act by blocking the nicotinic acetylcholine receptor. Lipoic acid is essential for aerobic metabolism in mammals and also has strong affinity with many metals including gold, molybdenum, and tungsten. Other 1,2-dithiolanes have relevance in nanomaterials such as gold nanoparticles or transition metal dichalcogenide monolayers (TMDs) (MoS2, MoS2 and Tungsten disulfide, WS2). Asparagusic-acid.png, asparagusic acid Nereistoxin.svg, nereistoxin, from which insecticides including cartap and bensultap were derived Lipoic acid.svg, lipoic acid 1,3-Di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dithiol
In organic chemistry, a dithiol is a type of organosulfur compound with two thiol () functional groups. Their properties are generally similar to those of monothiols in terms of solubility, odor, and volatility. They can be classified according to the relative location of the two thiol groups on the organic backbone. File:C6H4(SH)2.svg, Benzene-1,2-dithiol, the parent aromatic dithiol File:1,3-Propandithiol Structural Formula V1.svg, Propane-1,3-dithiol File:Meso-2,3-dimercaptosuccinic-acid-2D-skeletal-A-configurations-labelled.png, The drug meso-2,3-dimercaptosuccinic acid File:Dimercaprol.svg, Dimercaprol ("British anti-Lewisite"), an early antidote for arsenic poisoning File:Dihydrolipoic-acid-2D-skeletal.png, Dihydrolipoic acid, a vitamin File:Dithiothreitol.png, Dithiothreitol, a reagent in protein biochemistry Geminal dithiols Geminal dithiols have the formula RR'C(SH)2. They are derived from aldehydes and ketones by the action of hydrogen sulfide. Their stability con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl () group of an alcohol), and the word is a blend of "''thio-''" with "alcohol". Many thiols have strong odors resembling that of garlic or rotten eggs. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the "smell of natural gas" is due to the smell of the thiol used as the odorant. Thiols are sometimes referred to as mercaptans () or mercapto compounds, a term introduced in 1832 by William Christopher Zeise and is derived from the Latin ('capturing mercury')''Oxford American Dictionaries'' (Mac OS X Leopard). because the thiolate group () bonds very strong ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reagents For Organic Chemistry
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a substance ''consumed'' in the course of a chemical reaction. ''Solvents'', though involved in the reaction mechanism, are usually not called reactants. Similarly, ''catalysts'' are not consumed by the reaction, so they are not reactants. In biochemistry, especially in connection with enzyme-catalyzed reactions, the reactants are commonly called substrates. Definitions Organic chemistry In organic chemistry, the term "reagent" denotes a chemical ingredient (a compound or mixture, typically of inorganic or small organic molecules) introduced to cause the desired transformation of an organic substance. Examples include the Collins reagent, Fenton's reagent, and Grignard reagents. Analytical chemistry In analytical chemistry, a reagent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiols
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl () group of an alcohol), and the word is a blend of "''thio-''" with "alcohol". Many thiols have strong odors resembling that of garlic or rotten eggs. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the "smell of natural gas" is due to the smell of the thiol used as the odorant. Thiols are sometimes referred to as mercaptans () or mercapto compounds, a term introduced in 1832 by William Christopher Zeise and is derived from the Latin ('capturing mercury')''Oxford American Dictionaries'' (Mac OS X Leopard). because the thiolate group () bonds very strongly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dioxane
1,4-Dioxane () is a heterocyclic organic compound, classified as an ether. It is a colorless liquid with a faint sweet odor similar to that of diethyl ether. The compound is often called simply dioxane because the other dioxane isomers ( 1,2- and 1,3-) are rarely encountered. Dioxane is used as a solvent for a variety of practical applications as well as in the laboratory, and also as a stabilizer for the transport of chlorinated hydrocarbons in aluminum containers.Wisconsin Department of Health Services (20131,4-Dioxane Fact Sheet Publication 00514. Accessed 2016-11-12. Synthesis Dioxane is produced by the acid-catalysed dehydration of diethylene glycol, which in turn is obtained from the hydrolysis of ethylene oxide. In 1985, the global production capacity for dioxane was between 11,000 and 14,000 tons. In 1990, the total U.S. production volume of dioxane was between 5,250 and 9,150 tons. Structure The dioxane molecule is centrosymmetric, meaning that it adopts a chai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
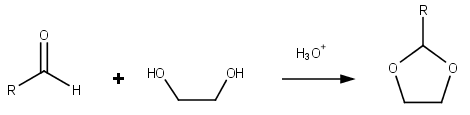
Dioxolane
Dioxolane is a heterocyclic acetal with the chemical formula (CH2)2O2CH2. It is related to tetrahydrofuran by interchange of one oxygen for a CH2 group. The corresponding saturated 6-membered C4O2 rings are called dioxanes. The isomeric 1,2-dioxolane (wherein the two oxygen centers are adjacent) is a peroxide. 1,3-dioxolane is used as a solvent and as a comonomer in polyacetals. As a class of compounds Dioxolanes are a group of organic compounds containing the dioxolane ring. Dioxolanes can be prepared by acetalization of aldehydes and ketalization of ketones with ethylene glycol. (+)-''cis''-Dioxolane is the trivial name for which is a muscarinic acetylcholine receptor agonist. Protecting groups Organic compounds containing carbonyl groups sometimes need protection so that they do not undergo reactions during transformations of other functional groups that may be present. A variety of approaches to protection and deprotection of carbonyls including as dioxolanes are known ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene Glycol
Ethylene glycol (IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes, as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odorless, colorless, flammable, viscous liquid. Ethylene glycol has a sweet taste, but it is toxic in high concentrations. Production Industrial routes Ethylene glycol is produced from ethylene (ethene), via the intermediate ethylene oxide. Ethylene oxide reacts with water to produce ethylene glycol according to the chemical equation: This reaction can be catalyzed by either acids or bases, or can occur at neutral pH under elevated temperatures. The highest yields of ethylene glycol occur at acidic or neutral pH with a large excess of water. Under these conditions, ethylene glycol yields of 90% can be achieved. The major byproducts are the oligomers diethylene glycol, triethylene glycol, and tetraethylene glycol. The separation of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diol
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified. The most common industrial diol is ethylene glycol. Examples of diols in which the hydroxyl functional groups are more widely separated include 1,4-butanediol and propylene-1,3-diol, or beta propylene glycol, . Synthesis of classes of diols Geminal diols A geminal diol has two hydroxyl groups bonded to the same atom. These species arise by hydration of the carbonyl compounds. The hydration is usually unfavorable, but a notable exception is formaldehyde which, in water, exists in equilibrium with methanediol H2C(OH)2. Another example is (F3C)2C(OH)2, the hydrated form of hexafluoroacetone. Many gem-diols undergo further condensation to give dimeric and oligomeric derivatives. This reaction applies to glyoxal and related aldehydes. Vicinal diols In a vicinal diol, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |