|
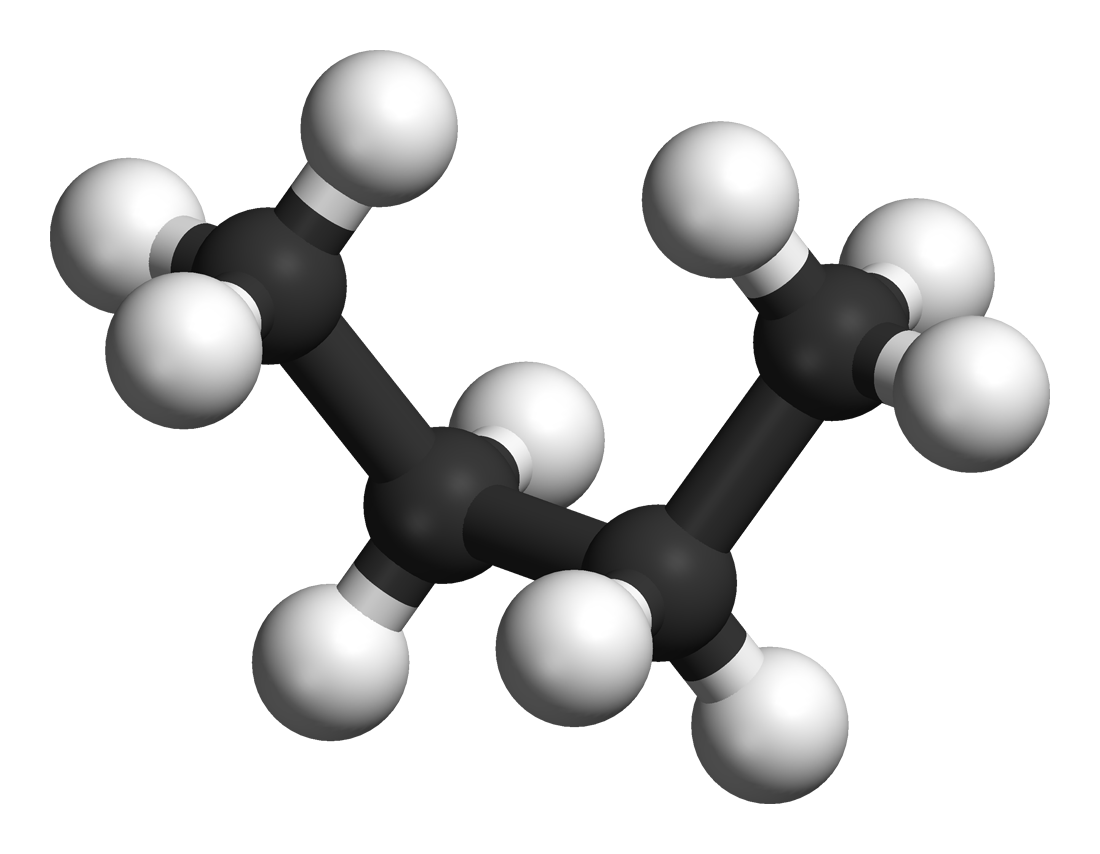
Eclipsed
In chemistry an eclipsed conformation is a conformation in which two substituents X and Y on adjacent atoms A, B are in closest proximity, implying that the torsion angle X–A–B–Y is 0°. Such a conformation can exist in any open chain, single chemical bond connecting two sp3- hybridised atoms, and it is normally a conformational energy maximum. This maximum is often explained by steric hindrance, but its origins sometimes actually lie in hyperconjugation (as when the eclipsing interaction is of two hydrogen atoms). In order to gain a deeper understanding of eclipsed conformations in organic chemistry, it is first important to understand how organic molecules are arranged around bonds, as well as how they move and rotate. In the example of ethane, two methyl groups are connected with a carbon-carbon sigma bond, just as one might connect two Lego pieces through a single “stud” and “tube”. With this image in mind, if the methyl groups are rotated around the bond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkane Stereochemistry
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by rotations about formally single bonds (refer to figure on single bond rotation). While any two arrangements of atoms in a molecule that differ by rotation about single bonds can be referred to as different conformations, conformations that correspond to local minima on the potential energy surface are specifically called conformational isomers or conformers. Conformations that correspond to local maxima on the energy surface are the transition states between the local-minimum conformational isomers. Rotations about single bonds involve overcoming a rotational energy barrier to interconvert one conformer to another. If the energy barrier is low, there is free rotation and a sample of the compound exists as a rapidly equilibrating mixture of multiple conformers; if the energy barrier is high enough then there is restricted rotation, a molecule may exist for a rela ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conformational Isomerism
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by rotations about formally single bonds (refer to figure on single bond rotation). While any two arrangements of atoms in a molecule that differ by rotation about single bonds can be referred to as different conformations, conformations that correspond to local minima on the potential energy surface are specifically called conformational isomers or conformers. Conformations that correspond to local maxima on the energy surface are the transition states between the local-minimum conformational isomers. Rotations about single bonds involve overcoming a rotational energy barrier to interconvert one conformer to another. If the energy barrier is low, there is free rotation and a sample of the compound exists as a rapidly equilibrating mixture of multiple conformers; if the energy barrier is high enough then there is restricted rotation, a molecule may exist for a rel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eclipsed Conformation
In chemistry an eclipsed conformation is a conformation in which two substituents X and Y on adjacent atoms A, B are in closest proximity, implying that the torsion angle X–A–B–Y is 0°. Such a conformation can exist in any open chain, single chemical bond connecting two sp3- hybridised atoms, and it is normally a conformational energy maximum. This maximum is often explained by steric hindrance, but its origins sometimes actually lie in hyperconjugation (as when the eclipsing interaction is of two hydrogen atoms). In order to gain a deeper understanding of eclipsed conformations in organic chemistry, it is first important to understand how organic molecules are arranged around bonds, as well as how they move and rotate. In the example of ethane, two methyl groups are connected with a carbon-carbon sigma bond, just as one might connect two Lego pieces through a single “stud” and “tube”. With this image in mind, if the methyl groups are rotated around the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Newman Projection
A Newman projection is a drawing that helps visualize the 3-dimensional structure of a molecule. This projection most commonly sights down a carbon-carbon bond, making it a very useful way to visualize the stereochemistry of alkanes. A Newman projection visualizes the conformation of a chemical bond from front to back, with the front atom represented by the intersection of three lines (a dot) and the back atom as a circle. The front atom is called ''proximal'', while the back atom is called ''distal''. This type of representation clearly illustrates the specific dihedral angle between the proximal and distal atoms. This projection is named after American chemist Melvin Spencer Newman, who introduced it in 1952 as a partial replacement for Fischer projections, which are unable to represent conformations and thus conformers properly.Newman, MS. ''Record. Chem. Progr. (Kresge-Hooker Sci. Lib.) 1952,'' 13'', 111'' This diagram style is an alternative to a sawhorse projection, wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strain (chemistry)
In chemistry, a molecule experiences strain when its chemical structure undergoes some stress which raises its internal energy in comparison to a strain-free reference compound. The internal energy of a molecule consists of all the energy stored within it. A strained molecule has an additional amount of internal energy which an unstrained molecule does not. This extra internal energy, or strain energy, can be likened to a compressed spring.Anslyn and Dougherty, ''Modern Physical Organic Chemistry'', University Science Books, 2006, Much like a compressed spring must be held in place to prevent release of its potential energy, a molecule can be held in an energetically unfavorable conformation by the bonds within that molecule. Without the bonds holding the conformation in place, the strain energy would be released. Summary Thermodynamics The equilibrium of two molecular conformations is determined by the difference in Gibbs free energy of the two conformations. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hyperconjugation
In organic chemistry, hyperconjugation (σ-conjugation or no-bond resonance) refers to the delocalization of electrons with the participation of bonds of primarily σ-character. Usually, hyperconjugation involves the interaction of the electrons in a sigma (σ) orbital (e.g. C–H or C–C) with an adjacent unpopulated non-bonding p or antibonding σ* or π* orbitals to give a pair of extended molecular orbitals. However, sometimes, low-lying antibonding σ* orbitals may also interact with filled orbitals of lone pair character (n) in what is termed '' negative hyperconjugation''. Increased electron delocalization associated with hyperconjugation increases the stability of the system. In particular, the new orbital with bonding character is stabilized, resulting in an overall stabilization of the molecule. Only electrons in bonds that are in the β position can have this sort of direct stabilizing effect — donating from a sigma bond on an atom to an orbital in another a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Staggered Conformation
In organic chemistry, a staggered conformation is a chemical conformation of an ethane-like moiety abcX–Ydef in which the substituents a, b, and c are at the maximum distance from d, e, and f; this requires the torsion angles to be 60°. It is the opposite of an eclipsed conformation, in which those substituents are as close to each other as possible. Such a conformation exists in any open chain single chemical bond connecting two sp3- hybridised atoms, and is normally a conformational energy minimum. For some molecules such as those of ''n''-butane, there can be special versions of staggered conformations called ''gauche'' and ''anti''; see first Newman projection diagram in Conformational isomerism. Crystal structures: The staggered/eclipsed configurations distinguish different crystalline structures of e.g. cubic/hexagonal boron nitride, and diamond Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quadruple Bond
A quadruple bond is a type of chemical bond between two atoms involving eight electrons. This bond is an extension of the more familiar types double bonds and triple bonds. Stable quadruple bonds are most common among the transition metals in the middle of the , such as rhenium, tungsten, technetium, molybdenum and chromium. Typically the ligands that support quadruple bonds are π-donors, not π-acceptors. History Chromium(II) acetate, Cr2(''μ''-O2CCH3)4(H2O)2, was the first chemical compound containing a quadruple bond to be synthesized. It was described in 1844 by E. Peligot, although its distinctive bonding was not recognized for more than a century. The first crystallographic study of a compound with a quadruple bond was provided by Soviet chemists for salts of . The very short Re–Re distance was noted. This short distance (and the salt's diamagnetism) indicated Re–Re bonding. These researchers however misformulated the anion as a derivative of Re(II), i.e., . Soo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethane Conformations And Relative Energies
Ethane ( , ) is an organic chemical compound with chemical formula . At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is isolated on an industrial scale from natural gas and as a petrochemical by-product of petroleum refining. Its chief use is as feedstock for ethylene production. Related compounds may be formed by replacing a hydrogen atom with another functional group; the ethane moiety is called an ethyl group. For example, an ethyl group linked to a hydroxyl group yields ethanol, the alcohol in beverages. History Ethane was first synthesised in 1834 by Michael Faraday, applying electrolysis of a potassium acetate solution. He mistook the hydrocarbon product of this reaction for methane and did not investigate it further. During the period 1847–1849, in an effort to vindicate the radical theory of organic chemistry, Hermann Kolbe and Edward Frankland produced ethane by the reductions of propionitrile ( eth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |