|
Divinyl Ketone
image:(1E,4E)-1,5-diphenylpenta-1,4-dien-3-one 200.svg, Dibenzylideneacetone is a well known dienone, 240px A dienone is a class of organic compounds with the general formula , where R is any substituent, but often H. They are formally "derived from 1,4-diene compounds by conversion of a –CH2– group into –C(=O)– group", resulting in "a Conjugated system, conjugated structure". They are a kind of enone. The class includes some heterocyclic compounds. Properties and occurrence The parent member is divinyl ketone. It is a colorless liquid (boiling point, b.p. 38-40 °C) that tends to polymerize upon standing. Dienones can arise via tautomerism of resorcinols and some 2-Hydroxypyridine, hydroxypyridines. Being multifunctional, dienones engage in many reactions. They are often good dienophiles. They function as ligands, forming metal-alkene complexes such as tris(dibenzylideneacetone)dipalladium(0). Cyclic dienones Extensive work has been reported on cyclic dienones ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tris(dibenzylideneacetone)dipalladium(0)
Tris(dibenzylideneacetone)dipalladium(0) or [Pd2(dba)3] is an organopalladium compound. The compound is a complex of palladium(0) with dibenzylideneacetone (dba). It is a dark-purple/brown solid, which is modestly soluble in organic solvents. Because the dba ligands are easily displaced, the complex is used as a homogeneous catalyst in organic synthesis. Preparation and structure First reported in 1970, it is prepared from dibenzylideneacetone and sodium tetrachloropalladate. Because it is commonly recrystallized from chloroform, the complex is often supplied as the adduct [Pd2(dba)3·CHCl3].Jiro Tsuji and Ian J. S. Fairlamb "Tris(dibenzylideneacetone)dipalladium–Chloroform" E-EROS, 2008. The purity of samples can be variable. In [Pd2(dba)3], the pair of Pd atoms are separated by 320 picometer, pm but are tied together by dba units. The Pd(0) centres are bound to the alkene parts of the dba ligands. Applications [Pd2(dba)3] is used as a source of soluble Pd(0), in parti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diketone
In organic chemistry, a dicarbonyl is a molecule containing two carbonyl () groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls are in close enough proximity that their reactivity is changed, such as 1,2-, 1,3-, and 1,4-dicarbonyls. Their properties often differ from those of monocarbonyls, and so they are usually considered functional groups of their own. These compounds can have symmetrical or unsymmetrical substituents on each carbonyl, and may also be functionally symmetrical (dialdehydes, diketones, diesters, ''etc.'') or unsymmetrical (keto-esters, keto-acids, ''etc.''). 1,2-Dicarbonyls 1,2-Dialdehyde The only 1,2-dialdehyde is glyoxal, . Like many alkyldialdehydes, glyoxal is encountered almost exclusively as its hydrate and oligomers thereof. These derivatives often behave equivalently to the aldehydes since hydration is reversible. Glyoxal condenses ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetraphenylcyclopentadienone
Tetraphenylcyclopentadienone is an organic compound with the formula . It is classified as a cyclic dienone. It is a dark purple to black crystalline solid that is soluble in organic solvents. It is an easily made building block for many organic and organometallic compounds. Structure The C5O core of the molecule is planar and conjugated, but the bonds have a definite alternating single- and double-bond nature. The C2–C3 and C4–C5 distances are 1.35 Å, while the C1–C2, C3–C4, C5–C1 are closer to single bonds with distances near 1.50 Å. The phenyl groups of tetraphenylcyclopentadienone adopt a "propeller" shape in its 3D Chemical structure, conformation. The four phenyl rings are rotated out of the plane of the central ring because of steric repulsion with each other. Unlike the parent compound cyclopentadienone, which rapidly dimerizes, the tetraphenyl derivative is isolable at room temperature. Synthesis Tetraphenylcyclopentadienone can be synthesized by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentadienone
Cyclopentadienone is an organic compound with molecular formula C5H4O. The parent cyclopentadienone is rarely encountered, because it rapidly dimerizes. Many substituted derivatives are known, notably tetraphenylcyclopentadienone. Such compounds are used as ligands in organometallic chemistry Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so .... left, The Knölker complex, derived from a substituted cyclopentadienone, is a catalyst for transfer hydrogenation. Preparation Cyclopentadienone can be generated by the photolysis or pyrolysis of various substances (e.g. 1,2-benzoquinone), and then isolated in an matrix isolation, argon matrix at . It dimerizes readily upon thawing the matrix at . See also * Dienone References {{Cyclopentadienide complexes Ketones Fully conjugat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (− O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule. Phenols are both synthesized industrially and produced by plants and microorganisms. Properties Acidity Phenols are more acidic than typical alcohols. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12). Deprotonation of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides (aryloxides, according to the IUPAC Gold Book). Condensation with aldehydes and ketones Phenols are susceptible to electrophilic aromatic substitutions. Condensation with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dienone–phenol Rearrangement
The dienone–phenol rearrangement is a Organic reaction, reaction in organic chemistry first reported in 1921 by Karl von Auwers and Karl Ziegler. A common example of dienone–phenol rearrangement is 4,4-disubstituted converting into a stable 3,4-disubstituted phenol in presence of acid. A similar rearrangement is possible with a 2,2-disubstituted cyclohexadienone to its corresponding disubstituted phenol. Usually this type of Rearrangement_reaction, rearrangement is spontaneous unless a dichloromethyl group is present at the 4th position or the process is otherwise blocked. Reaction mechanism The reaction mechanism of 4,4-disubstituted cyclohexadienones to 3,4-disubstituted phenol is illustrated here. : The migration tendency for the two different groups (R) present at either 4,4 position or 2,2 position can be determined by comparing the relative stability of the intermediate carbocation formed during rearrangement. In case of acid-promoted conditions, some relative migrat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinone
The quinones are a class of organic compounds that are formally "derived from aromatic compounds benzene.html" ;"title="uch as benzene">uch as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds", resulting in "a fully Conjugated system, conjugated cyclic diketone, dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone" (thus the name of the class). Other important examples are 1,2-benzoquinone (''ortho''-quinone), 1,4-naphthoquinone and 9,10-anthraquinone. The name is derived from that of quinic acid (with the suffix "-one" indicating a ketone), since it is one of the compounds obtained upon oxidation of quinic acid. Quinic acid, like quinine is obtained from cinchona bark, called quinaquina in the indigenous languages of Peruvian tribes. Properties Quinones are oxidized derivatives of aromatic compounds an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tropone
Tropone or 2,4,6-cycloheptatrien-1-one is an organic compound with some importance in organic chemistry as a non-benzenoid aromaticity, aromatic. The compound consists of a ring of seven carbon atoms with three conjugated system, conjugated alkene groups and a ketone group. The related compound tropolone (2-hydroxy-2,4,6-cycloheptatrien-1-one) has an additional Alcohol (chemistry), alcohol (or an enol including the double bond) group next to the ketone. Tropones are uncommon in natural products, with the notable exception of the 2-hydroxyl derivatives, which are called tropolones. Tropone has been known since 1951 and is also called ''cycloheptatrienylium oxide''. The name tropolone was coined by Michael J. S. Dewar, M. J. S. Dewar in 1945 in connection to perceived aromatic properties. Properties Dewar in 1945 proposed that tropones could have aromatic properties. The carbonyl group is more dielectric, polarized as a result of the triene ring, giving a partial positive charge o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
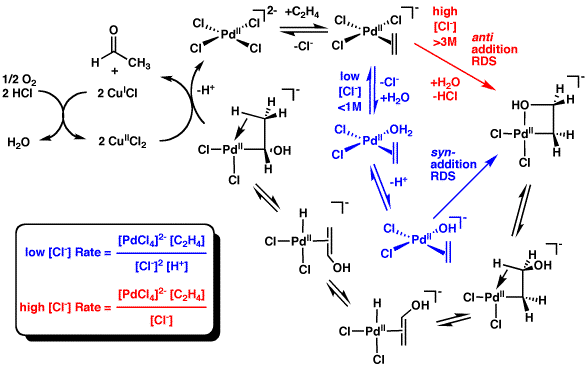
Metal-alkene Complex
In organometallic chemistry, a transition metal alkene complex is a coordination compound containing one or more alkene ligands. The inventory is large.Elschenbroich, C. "Organometallics" (2006) Wiley-VCH: Weinheim. Such compounds are intermediates in many catalytic reactions that convert alkenes to other organic products. Monoalkenes Complexes of ethylene are particularly common. Examples include Zeise's salt (see figure), Rh2Cl2(C2H4)4, Cp*2Ti(C2H4), and . Homoleptic alkene-complexes are well known but often are highly reactive. Examples include Ni(C2H4)3, o(C2H4)4sup>−, and e(C2H4)4sup>2−. Substituted monoalkenes are common ligands. Cyclooctene is found in chlorobis(cyclooctene)rhodium dimer. Alkenes with electron-withdrawing groups commonly bind strongly to low-valent metals. Examples of such ligands are TCNE, tetrafluoroethylene, maleic anhydride, and esters of fumaric acid. These acceptors form adducts with many zero-valent metals. Dienes, trienes, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |