|
Dimethylamine
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005. Structure and synthesis The molecule consists of a nitrogen atom with two methyl substituents and one hydrogen. Dimethylamine is a weak base and the pKa of the ammonium CH3--CH3 is 10.73, a value above methylamine (10.64) and trimethylamine (9.79). Dimethylamine reacts with acids to form salts, such as dimethylamine hydrochloride, an odorless white solid with a melting point of 171.5 °C. Dimethylamine is produced by catalytic reaction of methanol and ammonia at elevated temperatures and high pressure: : Natural occurrence Dimethylamine is found quite widely distributed in animals and plants, and is present in many foods at the level of a few mg/kg. Uses Dimethylamine is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylamine
Trimethylamine (TMA) is an organic compound with the formula N(CH3)3. It is a trimethylated derivative of ammonia. TMA is widely used in industry. At higher concentrations it has an ammonia-like odor, and can cause necrosis of mucous membranes on contact. At lower concentrations, it has a "fishy" odor, the odor associated with rotting fish. Physical and chemical properties TMA is a colorless, hygroscopic, and flammable tertiary amine. It is a gas at room temperature but is usually sold as a 40% solution in water. It is also sold in pressurized gas cylinders. TMA protonates to give the trimethylammonium cation. Trimethylamine is a good nucleophile, and this reactivity underpins most of its applications. Trimethylamine is a Lewis base that forms adducts with a variety of Lewis acids. Production Industry and laboratory Trimethylamine is prepared by the reaction of ammonia and methanol employing a catalyst: :3 CH3OH + NH3 → (CH3)3N + 3 H2O This reaction coproduces the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Secondary Amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of electrons. Amines can also exist as hetero cyclic compounds. Aniline is the simplest aromatic amine, consisting of a benzene ring bonded to an amino group. Amines are classified into three types: primary (1°), secondary (2°), and tertiary (3°) amines. Primary amines (1°) contain one alkyl or aryl substituent and have the general formula RNH2. Secondary amines (2°) have two alkyl or aryl groups attached to the nitrogen atom, with the general formula R2NH. Tertiary amines (3°) contain three substituent groups bonded to the nitrogen atom, and are represented by the formula R3N. The functional group present in primary amines is called the amino group. Classification of amines Amines can be classified according to the nature and number o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylamine
Trimethylamine (TMA) is an organic compound with the formula N(CH3)3. It is a trimethylated derivative of ammonia. TMA is widely used in industry. At higher concentrations it has an ammonia-like odor, and can cause necrosis of mucous membranes on contact. At lower concentrations, it has a "fishy" odor, the odor associated with rotting fish. Physical and chemical properties TMA is a colorless, hygroscopic, and flammable tertiary amine. It is a gas at room temperature but is usually sold as a 40% solution in water. It is also sold in pressurized gas cylinders. TMA protonates to give the trimethylammonium cation. Trimethylamine is a good nucleophile, and this reactivity underpins most of its applications. Trimethylamine is a Lewis base that forms adducts with a variety of Lewis acids. Production Industry and laboratory Trimethylamine is prepared by the reaction of ammonia and methanol employing a catalyst: :3 CH3OH + NH3 → (CH3)3N + 3 H2O This reaction coproduces the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylamine
Methylamine, also known as methanamine, is an organic compound with a formula of . This colorless gas is a derivative of ammonia, but with one hydrogen atom being replaced by a methyl group. It is the simplest primary amine. Methylamine is sold as a solution in methanol, ethanol, tetrahydrofuran, or water, or as the anhydrous gas in pressurized metal containers. Industrially, methylamine is transported in its anhydrous form in pressurized railcars and tank trailers. It has a strong odor similar to rotten fish. Methylamine is used as a building block for the synthesis of numerous other commercially available compounds. Industrial production Methylamine has been produced industrially since the 1920s (originally by Commercial Solvents Corporation for dehairing of animal skins). This was made possible by and his wife Eugenia who discovered amination of alcohols, including methanol, on alumina or kaolin catalyst after WWI, filed two patent applications in 1919 and published an a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethylamine
Diethylamine is an organic compound with the formula . It is classified as a secondary amine. It is a flammable, volatile weakly alkaline liquid that is miscible with most solvents. It is a colorless liquid, but commercial samples often appear brown due to impurities. It has a strong ammonia-like odor. Production and uses The alumina-catalyzed reaction makes diethylamine from ethanol and ammonia. Diethylamine is obtained together with ethylamine and triethylamine. Annual production of the three ethylamines was estimated in 2000 to be 80,000,000 kg. Diethylamine is used in the production of corrosion inhibitor ''N'',''N''- diethylaminoethanol, by reaction with ethylene oxide. It is also a precursor to a wide variety of other commercial products. It is also sometimes used in the illicit production of LSD. Organic chemistry As the most abundantly available secondary amine that is liquid at room temperature, diethylamine has been extensively deployed in chemical synthesis. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylacetamide
Dimethylacetamide (DMAc or DMA) is the organic compound with the formula CH3C(O)N(CH3)2. This colorless, water-miscible, high-boiling liquid is commonly used as a polar solvent in organic synthesis. DMA is miscible with most other solvents, although it is poorly soluble in aliphatic hydrocarbons. Synthesis and production DMA is prepared commercially by the reaction of dimethylamine with acetic anhydride or acetic acid. Dehydration of the salt of dimethylamine and acetic acid also furnishes this compound: : CH3CO2H·HN(CH3)2 → H2O + CH3CON(CH3)2 Dimethylacetamide can also be produced by the reaction of dimethylamine with methyl acetate. : The separation and purification of the product is carried out by multistage distillation in rectification columns. DMA is obtained with essentially quantitive (99%) yield referred to methyl acetate. Reactions and applications The chemical reactions of dimethylacetamide are typical of ''N'',''N''-disubstituted amides. Hydrolysis of the acyl- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylformamide
Dimethylformamide, DMF is an organic compound with the chemical formula . Its structure is . Commonly abbreviated as DMF (although this initialism is sometimes used for 2,5-dimethylfuran, dimethylfuran, or dimethyl fumarate), this colourless liquid is Miscibility, miscible with Water (molecule), water and the majority of organic liquids. DMF is a common solvent for chemical reactions. Dimethylformamide is odorless, but chemical purity, technical-grade or degraded samples often have a fishy smell due to impurity of dimethylamine. Dimethylamine degradation impurities can be removed by Sparging (chemistry), sparging samples with an inert gas such as argon or by sonication, sonicating the samples under reduced pressure. As its name indicates, it is structurally related to formamide, having two methyl groups in the place of the two hydrogens. DMF is a polar molecule, polar (hydrophilic) aprotic solvent with a high boiling point. It facilitates reactions that follow polar mechanisms, such ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Unsymmetrical Dimethylhydrazine
Unsymmetrical dimethylhydrazine (abbreviated as UDMH; also known as 1,1-dimethylhydrazine, heptyl or Geptil) is a chemical compound with the formula H2NN(CH3)2 that is primarily used as a rocket propellant. At room temperature, UDMH is a colorless liquid, with a sharp, fishy, ammonia-like smell typical of organic amines. Samples turn yellowish on exposure to air and absorb oxygen and carbon dioxide. It is miscible with water, ethanol, and kerosene. At concentrations between 2.5% and 95% in air, its vapors are flammable. It is not sensitive to shock. Symmetrical dimethylhydrazine (1,2-dimethylhydrazine) also exists, but it is not as useful. UDMH can be oxidized in air to form many different substances, including toxic ones. Synthesis In 1875, UDMH was first prepared by Emil Fischer, who discovered and named the class of hydrazines, by reducing N-Nitrosodimethylamine with zinc in boiling acetic acid. Fischer's student Edward Renouf later studied UDMH more extensively as part ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
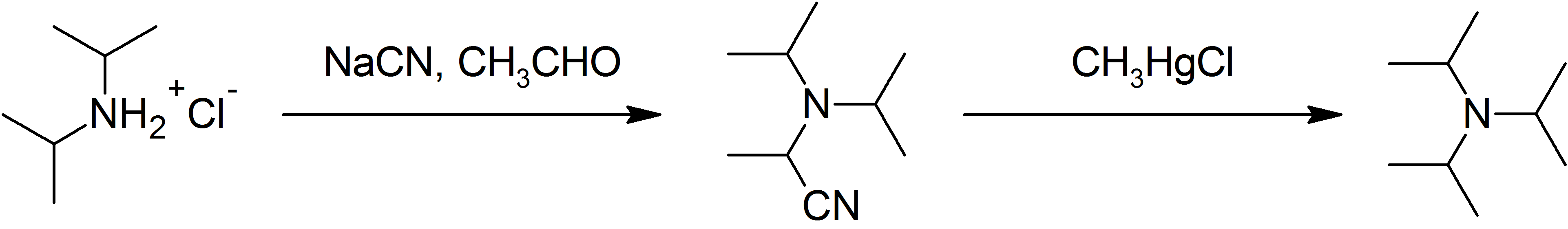
Triisopropylamine
Triisopropylamine is an organic chemical compound consisting of three isopropyl groups bound to a central nitrogen atom. As a hindered tertiary amine, it can be used as a non-nucleophilic base and as a stabilizer for polymers; however, its applications are limited by its relatively high cost and difficult synthesis. Structure In the early 1990s, theoretical studies and electron diffraction analysis of the 3D structure of the molecule, in the gas phase or in non-polar solvents, indicated that the bonds between the nitrogen atom and the three carbon atoms were nearly coplanar in the ground state, instead of forming a trigonal pyramid as in simpler amines. The average C-N-C angle was claimed to be 119.2°, much closer to the 120° of the flat configuration than to the 111.8° of trimethylamine. This peculiarity was attributed to steric hindrance by the bulky isopropyl radicals. However, in 1998 X-ray diffraction analysis of the crystallized solid showed that the C3N core is ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylaminopropylamine
Dimethylaminopropylamine (DMAPA) is a diamine used in the preparation of some surfactants, such as cocamidopropyl betaine which is an ingredient in many personal care products including soaps, shampoos, and cosmetics. BASF, a major producer, claims that DMAPA-derivatives do not sting the eyes and makes a fine-bubble foam, making it appropriate in shampoos. Preparation and reactions DMAPA is commonly produced commercially via the reaction between dimethylamine and acrylonitrile (a Michael reaction) to produce dimethylaminopropionitrile. A subsequent hydrogenation Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ... step yields DMAPA: : DMAPA is readily converted to the mustard dimethylaminopropyl-3-chloride, a powerful alkylating agent. Health effects Dimethylaminopropylamine is a kn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diisopropylamine
Diisopropylamine is a secondary amine with the chemical formula (Me2CH)2NH (Me = methyl). Diisopropylamine is a colorless liquid with an ammonia-like odor. Its lithium derivative, lithium diisopropylamide, known as LDA is a widely used reagent. Reactions and use Diisopropylamine is a common amine nucleophile in organic synthesis. Because it is bulky, it is a more selective nucleophile than other similar amines, such as dimethylamine. It reacts with organolithium reagents to give lithium diisopropylamide (LDA). LDA is a strong, non-nucleophilic base The main commercial applications of diisopropylamine is as a precursor to the herbicide, diallate and triallate as well as certain sulfenamides used in the vulcanization of rubber. It is also used to prepare ''N'',''N''-diisopropylethylamine (Hünig's base) by alkylation with diethyl sulfate. The bromide salt of diisopropylamine, diisopropylammonium bromide, is a room-temperature organic ferroelectric material. Preparation D ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. Like triethanolamine and the tetraethylammonium ion, it is often abbreviated TEA. It is a colourless volatile liquid with a strong fishy odor reminiscent of ammonia. Like diisopropylethylamine (Hünig's base), triethylamine is commonly employed in organic synthesis, usually as a base. Synthesis and properties Triethylamine is prepared by the alkylation of ammonia with ethanol: :NH3 + 3 C2H5OH → N(C2H5)3 + 3 H2O The pKa of protonated triethylamine is 10.75,David Evans Research Group and it can be used to prepare buffer solutions at that pH. The hydrochloride |