|
Diketene
Diketene is an organic compound with the molecular formula , and which is sometimes written as . It is formed by dimerization of ketene, . Diketene is a member of the oxetane family. It is used as a reagent in organic chemistry. It is a colorless liquid. Production Diketene is produced on commercial scale by dimerization of ketene. Reactions Heating or irradiation with UV light regenerates the ketene monomer: : Alkylated ketenes also dimerize with ease and form substituted diketenes. Diketene readily hydrolyzes in water forming acetoacetic acid. Its half-life in water is approximately 45 min. a 25 °C at . Certain diketenes with two aliphatic chains, such as alkyl ketene dimers (AKDs), are used industrially to improve hydrophobicity in paper. At one time acetic anhydride was prepared by the reaction of ketene with acetic acid: : ΔH = −63 kJ/mol Acetoacetylation Diketene also reacts with alcohols and amines to the corresponding acetoacetic acid deri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
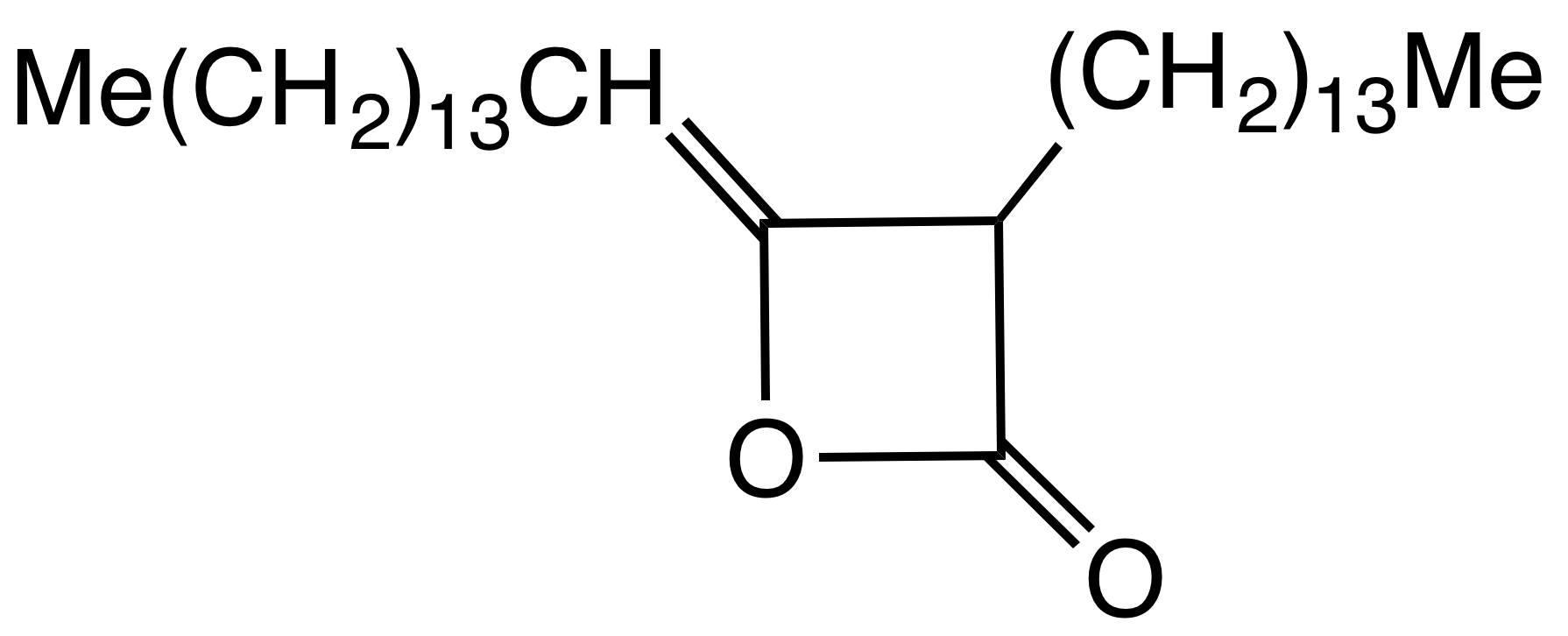
Alkyl Ketene Dimer
Alkyl ketene dimers (AKDs) are a family of organic compounds based on the 4-membered ring system of 2-oxetanone, oxetan-2-one, which is also the central structural element of propiolactone and diketene. Attached to the oxetane ring of technically relevant alkyl ketene dimers there is a C12 – C16 alkyl group in the 3-position and a C13 – C17 alkylidene group in the 4-position. The main application of alkylated ketene dimers is in the sizing of paper and cardboard, as well as in the hydrophobation of cellulose fibers. The products thus modified are distinguished by higher mechanical strengths and less penetration of water, inks or printing inks. AKD's feature hydrophobic alkyl groups extending from a beta-propiolactone ring. A specific example is derived from the dimerization of the ketene of stearic acid. This ketene is generated by pyrolysis of stearoyl chloride. AKD's react with the hydroxyl groups on the cellulose via esterification reaction. The esterification is competiti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetoacetic Acid
Acetoacetic acid ( IUPAC name: 3-oxobutanoic acid, also known as acetonecarboxylic acid or diacetic acid) is the organic compound with the formula CHCOCHCOOH. It is the simplest beta- keto acid, and like other members of this class, it is unstable. The methyl and ethyl esters, which are quite stable, are produced on a large scale industrially as precursors to dyes. Acetoacetic acid is a weak acid. Biochemistry Under typical physiological conditions, acetoacetic acid exists as its conjugate base, acetoacetate: : Unbound acetoacetate is primarily produced by liver mitochondria from its thioester with coenzyme A (CoA): : The acetoacetyl-CoA itself is formed by three routes: *3-hydroxy-3-methylglutaryl CoA releases acetyl CoA and acetoacetate: *: *Acetoacetyl-CoA can come from beta oxidation of butyryl-CoA: *: *Condensation of pair of acetyl CoA molecules as catalyzed by thiolase. *: In mammals, acetoacetate produced in the liver (along with the other two " ketone bodies") is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethenone
Ethenone is the formal name for ketene, an organic compound with formula or . It is the simplest member of the ketene class. It is an important reagent for acetylations. Properties Ethenone is a highly reactive gas (at Standard temperature and pressure, standard conditions) and has a sharp irritating odour. It is only reasonably stable at low temperatures (−80 °C). It must therefore always be prepared for each use and processed immediately, otherwise a dimerization to diketene occurs or it reacts to polymers that are difficult to handle. The polymer content formed during the preparation is reduced, for example, by adding sulfur dioxide to the ketene gas. Because of its cumulative double bonds, ethenone is highly reactive and reacts in an addition reaction H-acidic compounds to the corresponding acetic acid derivatives. It does for example react with water to acetic acid or with Primary amine, primary or secondary amines to the corresponding acetamides. Preparation Et ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stearic Acid
Stearic acid ( , ) is a saturated fatty acid with an 18-carbon chain. The IUPAC name is octadecanoic acid. It is a soft waxy solid with the formula . The triglyceride derived from three molecules of stearic acid is called stearin. Stearic acid is a prevalent fatty-acid in nature, found in many animal and vegetable fats, but is usually higher in animal fat than vegetable fat. It has a melting point of °C and a pKa of 4.50. Its name comes from the Greek word στέαρ "''stéar''", which means tallow. The salts and esters of stearic acid are called stearates. As its ester, stearic acid is one of the most common saturated fatty acids found in nature and in the food supply, following palmitic acid.Gunstone, F. D., John L. Harwood, and Albert J. Dijkstra "The Lipid Handbook with Cd-Rom. 3rd ed. Boca Raton: CRC Press, 2007. , Dietary sources of stearic acid include meat, poultry, fish, eggs, dairy products, and foods prepared with fats; beef tallow, lard, butterfat, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diarylide Pigment
Diarylide pigments are organic compounds that are used as pigments in inks and related materials. They often are yellow or yellow-green. To some extent, these organic compounds have displaced cadmium sulfide from the market. Being pigments, these compounds exist as (yellow) powders of low solubility in water. They are similar to the simpler monoazo pigments called arylide yellows.K. Hunger. W. Herbst "Pigments, Organic" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2012. Production and properties The formation of diarylide pigments involves the reaction of doubly diazotized aromatic diamines (derivatives of benzidine) with acetoacetanilide. By varying both of these components, several useful pigments have been produced. A related family of organic pigments are the simpler arylides, which arise from the coupling of ''mono''-diazonium salts with the same coupling partners. The pigments' colors can range from yellow to yellow-green. One common ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ullmann's Encyclopedia Of Industrial Chemistry
''Ullmann's Encyclopedia of Industrial Chemistry'' is a major reference work related to Chemical industry, industrial chemistry by chemist Fritz Ullmann, first published in 1914, and exclusively in German as "Enzyklopädie der Technischen Chemie" until 1984. History Ullmann's Encyclopedia of Industrial Chemistry is a major reference work related to industrial chemistry by chemist Fritz Ullmann. Its first edition was published in German by Fritz Ullmann in 1914. The fourth edition, published 1972 to 1984, already contained 25 volumes. The fifth edition, published 1985 to 1996, was the first version available in English. In 1997, the first online version was published. The year 2014 marked its Centennial, centenary. Ullmann's Encyclopedia was in its seventh edition, in 40 volumes, including one index volume and more than 1,050 articles (200 more than the sixth edition), approximately 30,000 pages, 22,000 images, 8,000 tables, 19,000 references and 85,000 indices. Editions * 19 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sizing
Sizing or size is a substance that is applied to, or incorporated into, other materials—especially papers and textiles—to act as a protective filler or glaze. Sizing is used in papermaking and textile manufacturing to change the absorption and wear characteristics of those materials. Sizing is used for oil-based surface preparation for gilding (sometimes called ''mordant'' in this context). It is used by painters and artists to prepare paper and textile surfaces for some art techniques. Sizing is used in photography to increase the sharpness of a print, to change the glossiness of a print, or for other purposes depending on the type of paper and printing technique. Fibers used in composite materials are treated with various sizing agents to promote adhesion with the matrix material. Sizing is used during paper manufacture to reduce the paper's tendency when dry to absorb liquid, with the goal of allowing inks and paints to remain on the surface of the paper and to dry t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thionyl Chloride
Thionyl chloride is an inorganic compound with the chemical formula . It is a moderately Volatility (chemistry), volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a Halogenation, chlorinating reagent, with approximately per year being produced during the early 1990s, but is occasionally also used as a solvent. It is toxic, reacts with water, and is also List of Schedule 3 substances (CWC), listed under the Chemical Weapons Convention as it may be used for the production of chemical weapons. Thionyl chloride is sometimes confused with sulfuryl chloride, , but the properties of these compounds differ significantly. Sulfuryl chloride is a source of chlorine whereas thionyl chloride is a source of chloride ions. Production The major industrial synthesis involves the reaction of sulfur trioxide and sulfur dichloride. This synthesis can be adapted to the laboratory by heating oleum to slowly distill the sulfur trioxide into a cooled fla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. Like triethanolamine and the tetraethylammonium ion, it is often abbreviated TEA. It is a colourless volatile liquid with a strong fishy odor reminiscent of ammonia. Like diisopropylethylamine (Hünig's base), triethylamine is commonly employed in organic synthesis, usually as a base. Synthesis and properties Triethylamine is prepared by the alkylation of ammonia with ethanol: :NH3 + 3 C2H5OH → N(C2H5)3 + 3 H2O The pKa of protonated triethylamine is 10.75,David Evans Research Group and it can be used to prepare buffer solutions at that pH. The hydrochloride |