|
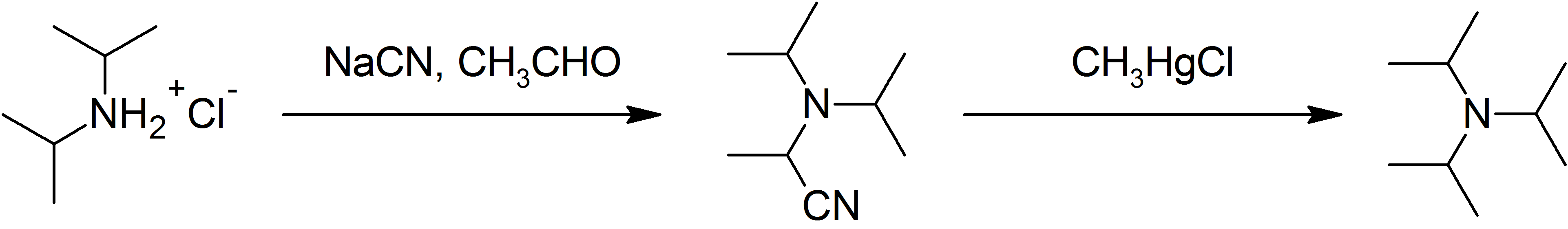
Diisopropylamine
Diisopropylamine is a secondary amine with the chemical formula (Me2CH)2NH (Me = methyl). Diisopropylamine is a colorless liquid with an ammonia-like odor. Its lithium derivative, lithium diisopropylamide, known as LDA is a widely used reagent. Reactions and use Diisopropylamine is a common amine nucleophile in organic synthesis. Because it is bulky, it is a more selective nucleophile than, say, dimethylamine. It reacts with organolithium reagents to give lithium diisopropylamide (LDA). LDA is a strong, non-nucleophilic base The main commercial applications of diisopropylamine is as a precursor to two herbicides, diallate and triallate, as well as certain sulfenamides used in the vulcanization of rubber. It is also used to prepare N,N-Diisopropylethylamine (Hünig's base) by alkylation with diethyl sulfate. The bromide salt of diisopropylamine, diisopropylammonium bromide, is a room-temperature organic ferroelectric material. Preparation Diisopropylamine, which is comm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylamine
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005. Structure and synthesis The molecule consists of a nitrogen atom with two methyl substituents and one proton. Dimethylamine is a weak base and the pKa of the ammonium CH3--CH3 is 10.73, a value above methylamine (10.64) and trimethylamine (9.79). Dimethylamine reacts with acids to form salts, such as dimethylamine hydrochloride, an odorless white solid with a melting point of 171.5 °C. Dimethylamine is produced by catalytic reaction of methanol and ammonia at elevated temperatures and high pressure: :2 CH3OH + NH3 → (CH3)2NH + 2 H2O Natural occurrence Dimethylamine is found quite widely distributed in animals and plants, and is present in many foods at the level ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
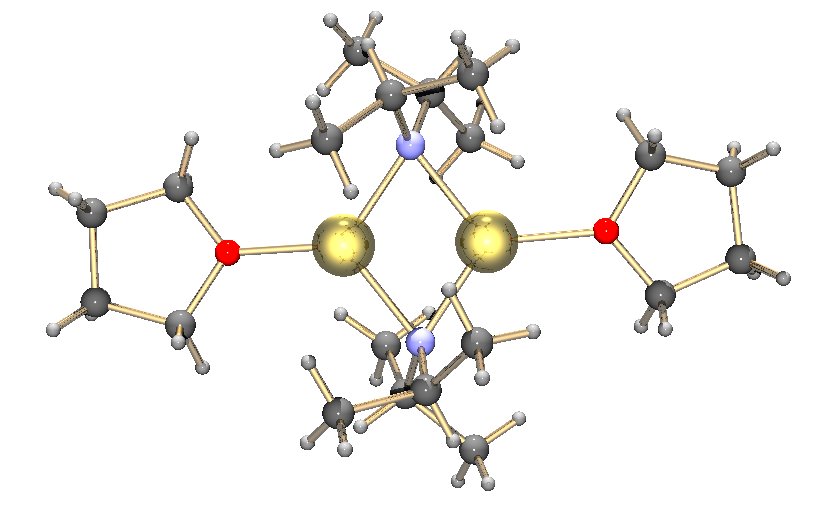
Lithium Diisopropylamide
Lithium diisopropylamide (commonly abbreviated LDA) is a chemical compound with the molecular formula . It is used as a strong base and has been widely utilized due to its good solubility in non-polar organic solvents and non-nucleophilic nature. It is a colorless solid, but is usually generated and observed only in solution. It was first prepared by Hamell and Levine in 1950 along with several other hindered lithium diorganylamides to effect the deprotonation of esters at the α position without attack of the carbonyl group. Preparation and structure LDA is commonly formed by treating a cooled (0 to −78 °C) mixture of tetrahydrofuran and diisopropylamine with ''n''-butyllithium. When dissociated, the diisopropylamide anion can become protonated to form diisopropylamine. Diisopropylamine has a p''K''a value of 36. Therefore, its conjugate base is suitable for the deprotonation of compounds with greater acidity, importantly, such weakly acidic compounds (carbon acids) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylamine
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005. Structure and synthesis The molecule consists of a nitrogen atom with two methyl substituents and one proton. Dimethylamine is a weak base and the pKa of the ammonium CH3--CH3 is 10.73, a value above methylamine (10.64) and trimethylamine (9.79). Dimethylamine reacts with acids to form salts, such as dimethylamine hydrochloride, an odorless white solid with a melting point of 171.5 °C. Dimethylamine is produced by catalytic reaction of methanol and ammonia at elevated temperatures and high pressure: :2 CH3OH + NH3 → (CH3)2NH + 2 H2O Natural occurrence Dimethylamine is found quite widely distributed in animals and plants, and is present in many foods at the level ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethylamine
Diethylamine is an organic compound with the formula (CH3CH2)2NH. It is a secondary amine. It is a flammable, weakly alkaline liquid that is miscible with most solvents. It is a colorless liquid, but commercial samples often appear brown due to impurities. It has a strong ammonia-like odor. Production and uses Diethylamine is made by the alumina-catalyzed reaction of ethanol and ammonia. It is obtained together with ethylamine and triethylamine. Annual production of three ethylamines was estimated in 2000 to be 80,000,000 kg. It is used in the production of corrosion inhibitor ''N'',''N''- diethylaminoethanol, by reaction with ethylene oxide. It is also a precursor to a wide variety of other commercial products. Diethylamine is also sometimes used in the illicit production of LSD. Supramolecular structure Diethylamine is the smallest and simplest molecule that features a supramolecular helix as its lowest energy aggregate. Other similarly sized hydrogen-bonding In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triisopropylamine
Triisopropylamine is an organic chemical compound consisting of three isopropyl groups bound to a central nitrogen atom. As a hindered tertiary amine, it can be used as a non-nucleophilic base and as a stabilizer for polymers; however, its applications are limited by its relatively high cost and difficult synthesis. Structure Triisopropylamine is notable as being among the most sterically hindered amines currently known. The even more crowded tri-''tert''-butylamine (''t''Bu3N) has never been synthesized, although ''ab initio'' quantum chemical computations as well as the existence of the even more crowded 2,2,4,4-tetramethyl-3-''t''-butyl-pentane-3-ol (tri-''tert''-butylcarbinol, ''t''Bu3COH) implies that it should be a stable molecule if it could be prepared. To date, di-''tert''-butyl(isopropyl)amine (''t''Bu2iPrN) has been prepared in low yield, as have a handful of tri-''tert''-alkylamines in which two of the ''tert''-alkyl groups are tied together in a ring, but the auth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butterworth-Heinemann
Butterworth–Heinemann is a British publishing company specialised in professional information and learning materials for higher education and professional training, in printed and electronic forms. It was formed in 1990 by the merger of Heinemann Professional Publishing and Butterworths Scientific, both subsidiaries of Reed International. With its earlier constituent companies, the founding dates back to 1923. It has publishing units in Oxford (UK) and Waltham, Massachusetts (United States). As of 2006, it is an imprint of Elsevier. See also *LexisNexis Butterworths LexisNexis is a part of the RELX corporation that sells data analytics products and various databases that are accessed through online portals, including portals for computer-assisted legal research (CALR), newspaper search, and consumer informa ... References External links * Book publishing companies of the United Kingdom Elsevier imprints {{publish-corp-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature, and must be prepared from compounds. Sodium is the sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been leached by the action of water from the Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans. Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide ( lye) is used in soap manufacture, and sodium chloride ( edible salt) is a de-icing agent and a nutrient for animals i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash. Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which exploit its caustic nature and its reactivity toward acids. An estimated 700,000 to 800,000 tonnes were produced in 2005. KOH is noteworthy as the precursor to most soft and liquid soaps, as well as numerous potassium-containing chemicals. It is a white solid that is dangerously corrosive. Properties and structure KOH exhibits high thermal stability. Because of this high stability and relatively low melting point, it is often melt-cast as pellets or rods, forms that have low surface area and convenient handling properties. These pellets become tacky in air because KOH is hygroscopic. Most commercial samples are ca. 90% pure, the remainder being water and carbonates. Its dissolution in water is strongly exothermic. Concentrated aqueous so ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkylamines
{{disambiguation ...
Alkylamines may refer to: * Aliphatic amine * Psychotropic alkylamines Psychotropic alkylamines are alkylamines that share the critical property of not containing an aromatic nucleus, but are still biologically active. While many of these molecules are stimulants (some of them natural), others are antiviral, have co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferroelectric
Ferroelectricity is a characteristic of certain materials that have a spontaneous electric polarization that can be reversed by the application of an external electric field. All ferroelectrics are also piezoelectric and pyroelectric, with the additional property that their natural electrical polarization is reversible. The term is used in analogy to ferromagnetism, in which a material exhibits a permanent magnetic moment. Ferromagnetism was already known when ferroelectricity was discovered in 1920 in Rochelle salt by Joseph Valasek.See and Thus, the prefix ''ferro'', meaning iron, was used to describe the property despite the fact that most ferroelectric materials do not contain iron. Materials that are both ferroelectric ''and'' ferromagnetic are known as multiferroics. Polarization When most materials are electrically polarized, the polarization induced, ''P'', is almost exactly proportional to the applied external electric field ''E''; so the polarization is a linear ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper Chromite
Copper chromite is an inorganic compound with the formula Cu2Cr2O5. It is a black solid that is used to catalyze reactions in organic synthesis. History The material was first described in 1908. The catalyst was developed in North America by Homer Burton Adkins and Wilbur Arthur Lazier, partly based on interrogation of German chemists after World War II in relation to the Fischer–Tropsch process. For this reason it is sometimes referred to as the Adkins catalyst or the Lazier catalyst. Chemical structure The compound adopts a spinel structure. The oxidation states for the constituent metals are Cu(II) and Cr(III). A variety of compositions are recognized for the substance, including Cu2CrO4·CuO·BaCrO4 ( CAS# 99328-50-4) and Cu2Cr2O5 (CAS# 12053-18-8). Commercial samples often contain barium oxide and other components. Production Copper chromite is produced by thermal decomposition of one of three substances. The traditional method is by the ignition of copper chromate: : 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper Oxide
Copper oxide is a compound from the two elements copper and oxygen. Copper oxide may refer to: * Copper(I) oxide (cuprous oxide, Cu2O) * Copper(II) oxide Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu2O or copper(I) oxide (cuprous oxide). As a mineral, it is known as tenorite. It ... (cupric oxide, CuO) * Copper peroxide (CuO2) * Copper(III) oxide (Cu2O3) * Copper(IV) oxide (CuO2) References {{Authority control Copper compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |